The following two cracking reactions for n-pentane occur in parallel: At 183C and 0.5 bar, both these
Question:
The following two cracking reactions for n-pentane occur in parallel: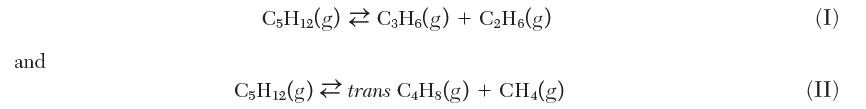
At 183°C and 0.5 bar, both these reactions contribute to the equilibrium composition of species in the system. For every 1.0 mole of n-pentane gas initially fed, 0.10 mol of propylene (C3H6) is formed at equilibrium from Reaction I. Calculate the equilibrium amount of trans-2-butene formed per mol of pentane fed. You may assume that Dhrxn for each reaction does not change with temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





