The following vaporliquid equilibrium data have been reported for a binary mixture of acetone (1) in water
Question:
The following vapor–liquid equilibrium data have been reported for a binary mixture of acetone (1) in water (2) at 1 atm. Test these data for thermodynamic consistency.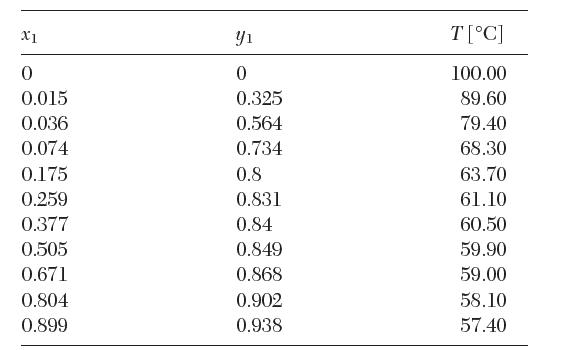
Transcribed Image Text:
X1 0 0.015 0.036 0.074 0.175 0.259 0.377 0.505 0.671 0.804 0.899 Y 0 0.325 0.564 0.734 0.8 0.831 0.84 0.849 0.868 0.902 0.938 T[C] 100.00 89.60 79.40 68.30 63.70 61.10 60.50 59.90 59.00 58.10 57.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use ThermoSolver to fi nd the activity coeffi cient model parameters for the data presented in Problems 8.55 and 8.56. Problems 8.55 The following vaporliquid equilibrium data have been reported for...
-
The following vaporliquid equilibrium data have been reported for a binary mixture of acetone (1) in chloroform (2) at 35. 17C. Test these data for thermodynamic consistency. X1 0 0.0821 0.1953...
-
A phase diagram for a binary mixture of ammonia (1) water (2) at 1 atm is shown in the following fi gure. Answer the following questions. Illustrate whenever possible the information you obtain from...
-
For each polynomial function, find (a) (-1), (b) (2), and (c) (0). f(x)=x5x4
-
After resolving their dispute, the two brothers encountered in Question 3-2 decide to resume their tax-accounting practice according to the terms of their original agreement. Again a dispute arises,...
-
Design a decimator that downsamples an input signal x(n) by a factor D = 5. Use the Remez algorithm to determine the coefficients of the FIR filter that has 0.1-dB ripple in the passband {0 /5} and...
-
Why do we need to perform EDA? Why should not we simply proceed directly to the modeling phase and start applying our high-powered data mining software?
-
You are product planner for product A (in problem 14.14 and figure 14.16). the field service manager, Al Trostel, has just called and told you that the requirements for B and F should each be...
-
Preparing a direct materials budget Each unit requires 3 l b s . of DM DM costs $ 0 . 6 0 per lb . Miles will begin the year with 2 0 , 0 0 0 l b s . of DM , but 3 0 , 0 0 0 l b s . at the end of...
-
You wish to fi t the benzene (1)isooctane (2) system to the following model for gE: The system temperature of interest is 200C. After a literature search, the only vaporliquid equilibrium data at...
-
Test the liquidvapor equilibrium data for the binary system of methanol (1)water (2) at 40C presented in Problem 8.53 for thermodynamic consistency by using the area test. Problem 8.53 Liquidvapor...
-
Classify the following items as (a) accrued revenue, (b) accrued expense, (c) unearned revenue, or (d) prepaid expense: 1. Bill for ads that appeared in prior month's local newspaper. 2. Fees...
-
1) Factor the following Expressions (Write your factors only, don't show your work) a) 2x - 32 = c) 3x-2x-8= b) 2x-6x-8=
-
Bloomfield Inc. manufactures widgets. A major piece of equipment used to make the widget is nearing the end of its useful life. The company is trying to decide whether they should lease new equipment...
-
1. a. What is network management? Illustrate network management functional flowchart. [2.5] b. What encoding and decoding mechanisms are used in fast Ethernet and gigabit Ethernet? What is meant by...
-
Project Data: Sam Parker owns and operates a consulting firm called Business Solutions. The business began operating in October 202X. Transactions for October and November 202X have been recorded and...
-
3. Use Hooke's law to predict which one out of each pair vibrates at a higher wavenumber. Explain your answer. (7 points) a) C-H and C-D* b) C-C and C=C where: 1 k v = 2, v=wavenumber c = velocity of...
-
How have capital, labor, and total factor productivity contributed to U.S. real GDP growth?
-
Chris Zulliger was a chef at the Plaza Restaurant in the Snowbird Ski Resort in Utah. The restaurant is located at the base of a mountain. As a chef for the Plaza, Zulliger was instructed by his...
-
Describe reasonable syntheses of Benzophenone, from each of the following starting materials and any necessary inorganic reagents. (a) Benzoyl chloride and benzene (b) Benzyl alcohol and bromobenzene...
-
The sex attractant of the female winter moth has been identified as the tetraene CH3(CH2)8CHCHCH2CHCHCH2CHCHCHCH2. Devise a synthesis of this material from 3, 6-hexadecadien-1-ol and allyl alcohol.
-
Hydrolysis of a compound a in dilute aqueous hydrochloric acid gave (along with methanol) a compound B, mp 164165C. Compound B had the molecular formula C16H16O4; it exhibited hydroxyl absorption in...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


