A phase diagram for a binary mixture of ammonia (1) water (2) at 1 atm is
Question:
A phase diagram for a binary mixture of ammonia (1) – water (2) at 1 atm is shown in the following fi gure. Answer the following questions. Illustrate whenever possible the information you obtain from the diagram:
(a) What is the lowest temperature that only liquid can exist? What is the composition?
(b) What is the highest temperature that only liquid can exist? What is the composition?
(c) Consider a mixture of 1 mole NH3 and 4 moles H2O at 193.15 K. What phases exist? What are their compositions? How many moles of each phase are present.
(d) What is the activity coeffi cient of NH3 in a liquid in equilibrium with its vapor at a temperature of 80°C. Use the Lewis/Randall reference state.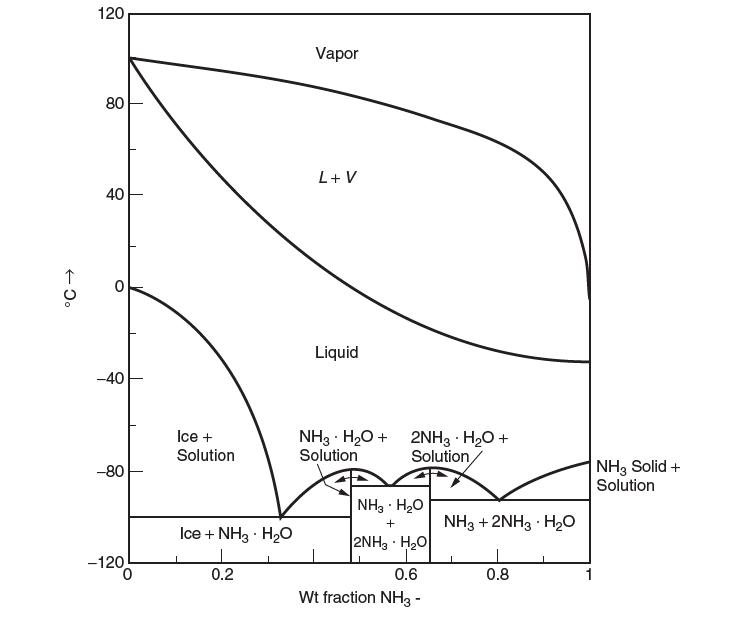
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





