A well-insulated container has 1.0 kg ice and 1.0 kg liquid water at 0C in equilibrium. 1.0
Question:
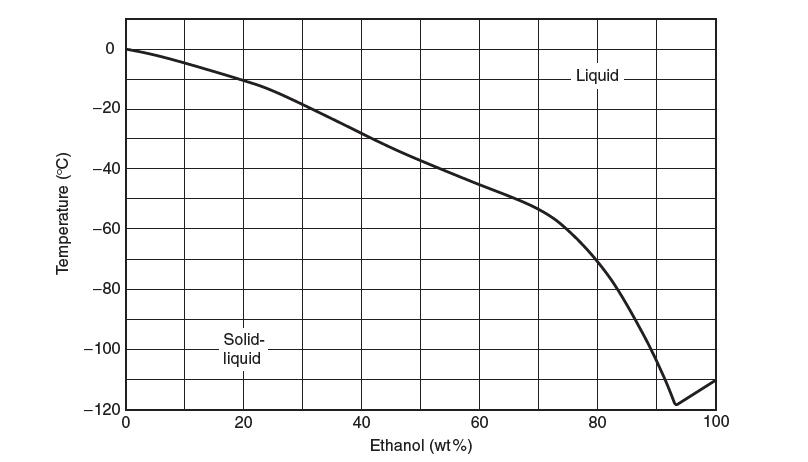
A well-insulated container has 1.0 kg ice and 1.0 kg liquid water at 0°C in equilibrium. 1.0 kg of liquid ethanol at 0°C is added to the system. At equilibrium, what is the fi nal state of the system?
A phase diagram for the binary mixture is shown in the following fi gure. The enthalpy of fusion for water is 6.01 kJ/mol and for ethanol is 5.02 kJ/mol. The enthalpy of mixing between water (1) and ethanol (2) has been reported to be fi t by: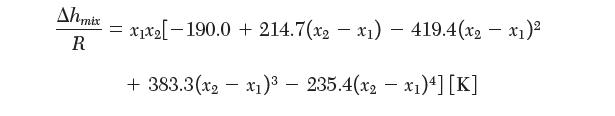
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





