Use the van der Waals equation of state to plot the inversion line for N2 on a
Question:
Use the van der Waals equation of state to plot the inversion line for N2 on a PT diagram, as schematically shown in Figure 5.10.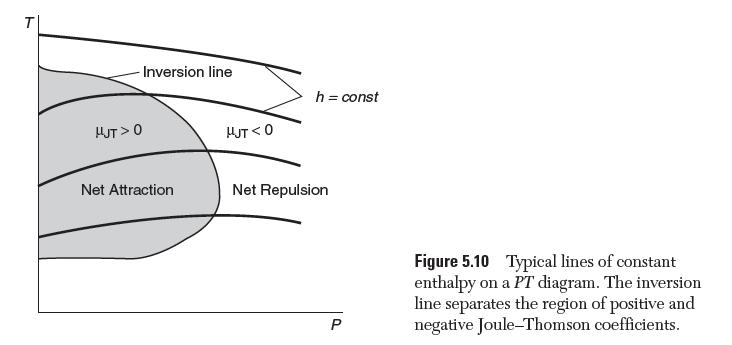
Transcribed Image Text:
T -Inversion line HJT> 0 Net Attraction HUT <0 h = const Net Repulsion P Figure 5.10 Typical lines of constant enthalpy on a PT diagram. The inversion line separates the region of positive and negative Joule-Thomson coefficients.
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
The Joule-Thomson coefficient, , given by is a function of temperature. The temperature at which = 0 is known as the inversion temperature. a. Use the van der Waals equation of state to determine...
-
Now assume that there are 1,500 identical firms in this competitive industry. That is, there are 1,500 firms, each of which has the cost data shown in the table. Complete the industry supply schedule...
-
Simplify each expression in Problems 722. Classify each answer by number of terms and degree. (x + 2y3z) - (x - 5y + 4z)
-
For this assignment you are going to construct a basic break-even analysis spreadsheet following the directions from this blog on techrepublic.com:...
-
Durban Metal Products, Ltd., of the Republic of South Africa makes specialty metal parts used in applications ranging from the cutting edges of bulldozer blades to replacement parts for Land Rovers....
-
Describe the Manage the Stage principle in a PRINCE2 project. AppendixLO1
-
Myles Company expects to produce 1,200,000 units of Product XX in 2012. Monthly production is expected to range from 80,000 to 120,000 units. Budgeted variable manufacturing costs per unit are:...
-
Marvin Company is a subsidiary of Hughes Corp. The controller believes that the yearly allowance for doubtful accounts for Marvin should be 8% of gross a ccounts receivable. Given the recession and...
-
Ethylene is liquefi ed by a JouleThomson expansion. It enters the throttling process at 50 bar and 0C and leaves at 10 bar. What is the fraction of the inlet stream that is liquefi ed?
-
Determine JT for steam at 1 MPa and 300C using data from the steam tables.
-
1. Describe at least four advantages of decentralization. Also describe at least two disadvantages to decentralization. 2. Compare and contrast a cost center, a revenue center, a profit center, and...
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Paul Petersen lives in Northern California. He owns a BMW car worth about $20,000. He wants to take a trip to Nevada with his girlfriend Patricia, who lives in Los Angeles. He takes his car into...
-
Do you see gendered patterns of interaction in personal relationships? Does knowing about gender linked patterns affect how other interpret on what happens in a relationships?
-
Significance For bone density scores that are normally distributed with a mean of 0 and a standard deviation of 1, find the percentage of scores that are significantly high (or at least 2 standard...
-
Under what conditions might it be appropriate to use a product-diminishing feature to accomplish price segmentation?
-
Quality Chicken grows and processes chickens. Each chicken is disassembled into five main parts. Information pertaining to production in July 2012 is: Joint cost of production in July 2012 was $50. A...
-
Use eqn 19.27 to deduce expressions for (a) The root mean square separation of the ends of the chain, (b) The mean separation of the ends, and (c) Their most probable separation. Evaluate these three...
-
Evaluate the radius of gyration, Rg, of (a) A solid sphere of radius a, (b) A long straight rod of radius a and length I. Show that in the case of a solid sphere of specific volume v" Rg/nm =...
-
Calculate the excluded volume in terms of the molecular volume on the basis that the molecules are spheres of radius a. Evaluate the osmotic virial coefficient in the case of bushy stunt virus, a =...
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


