Question: The Joule-Thomson coefficient, , given by is a function of temperature. The temperature at which = 0 is known as the inversion temperature. a.
The Joule-Thomson coefficient, μ, given by
![= T . || - - -[1 - Ta]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/4/6836553670be66631699964680686.jpg)
is a function of temperature. The temperature at which μ = 0 is known as the inversion temperature.
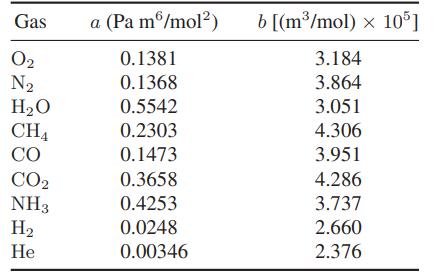
a. Use the van der Waals equation of state to determine the inversion temperature of H2, O2, N2, CO and CH4. The van der Waals parameters for these gases can be found in Table 6.4-1.
Table 6.4-1.
b. Repeat part (a) using the Redlich-Kwong equation of state.
= T . || - - -[1 - Ta]
Step by Step Solution
★★★★★
3.32 Rating (152 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


