Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please post step by step explanation An LPG (liquefied petroleum gas containing 19 mole % propane, 74 mole % 'n VPG (vloeibare petroleumgas wat uit
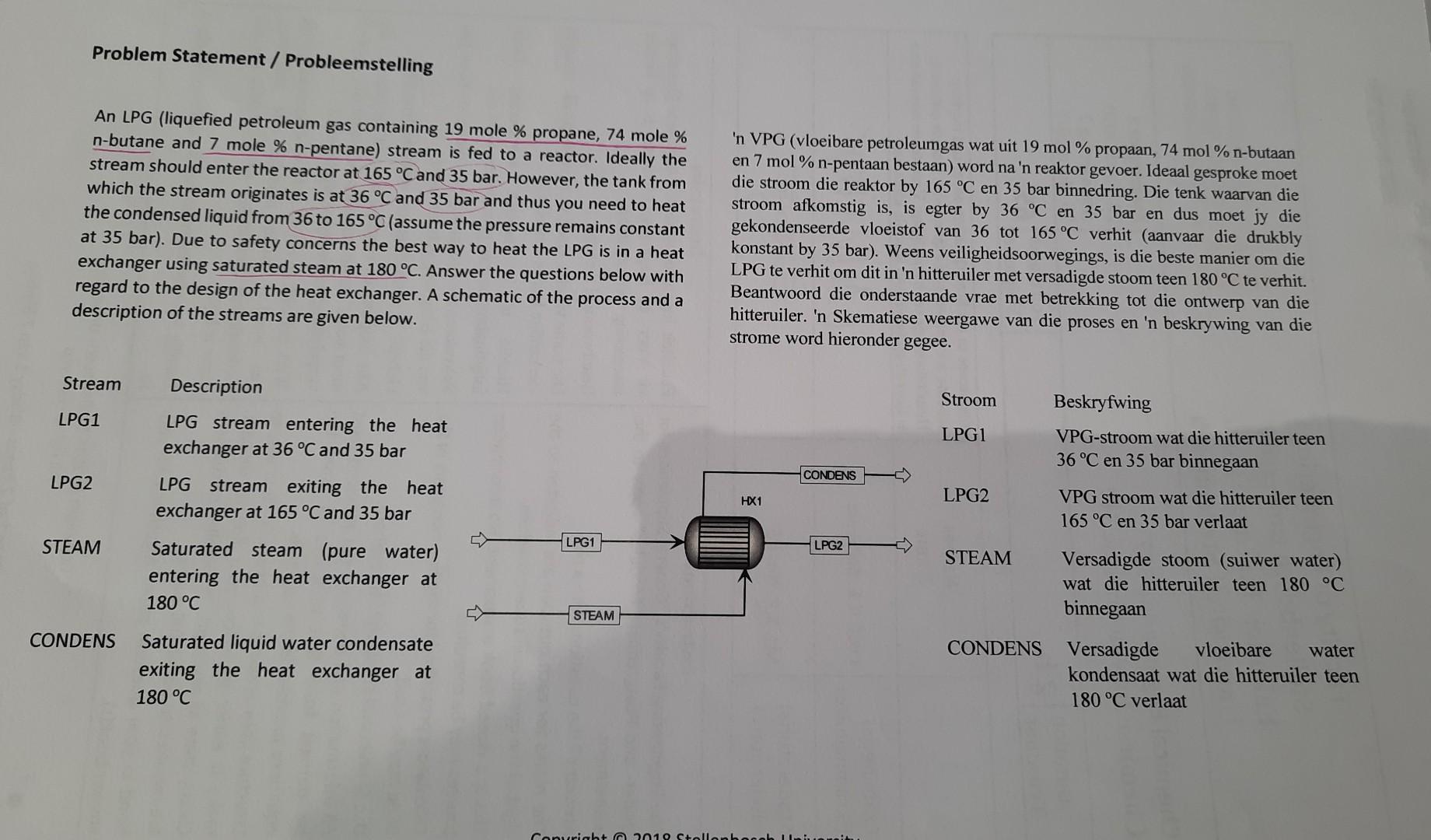

please post step by step explanation
An LPG (liquefied petroleum gas containing 19 mole \% propane, 74 mole \% 'n VPG (vloeibare petroleumgas wat uit 19 mol \% propaan, 74 mol \% n-butaan n-butane and 7 mole \% n-pentane) stream is fed to a reactor. Ideally the en 7mol% n-pentaan bestaan) word na'n reaktor gevoer. Ideaal gesproke moet stream should enter the reactor at 165C and 35 bar. However, the tank from die stroom die reaktor by 165C en 35 bar binnedring. Die tenk waarvan die which the stream originates is at 36C and 35 bar and thus you need to heat stroom afkomstig is, is egter by 36C en 35 bar en dus moet jy die the condensed liquid from 36 to 165C (assume the pressure remains constant gekondenseerde vloeistof van 36 tot 165C verhit (aanvaar die drukbly at 35 bar). Due to safety concerns the best way to heat the LPG is in a heat konstant by 35 bar). Weens veiligheidsoorwegings, is die beste manier om die exchanger using saturated steam at 180C. Answer the questions below with LPG te verhit om dit in 'n hitteruiler met versadigde stoom teen 180C te verhit. regard to the design of the heat exchanger. A schematic of the process and a Beantwoord die onderstaande vrae met betrekking tot die ontwerp van die description of the streams are given below. hitteruiler. 'n Skematiese weergawe van die proses en ' n beskrywing van die strome word hieronder gegee. Question 1 / Vraag 1 Use the van der Waals equation of state in combination with the van der Waals mixing rules to describe the PVT behaviour of the LPG and estimate the amount of heat that is required per mole LPG. Do so by answering the following questions: a) Prove that the following general relationship exists for the [10] molar enthalpy of a stream dH=CpdT+(VT(TV)P)dP b) Prove that the ideal gas enthalpy is pressure independent [3] (You can use any of the above derived equations, or any information given in the appendix) c) Determine an expression for the residual enthalpy of a fluid [17] that can be described by the van der Waals EOS. First prove the equation below and then apply it to the van der Waals equation of state. HR=T,V=T,V(V(VP)T+T(TP)V)dV d) Calculate the amount of heat required to heat a mole of this [15] LPG from 36C to 165C at a constant pressure of 35 bar. The following equation is available for the enthalpy of the gas mixture at 10Pa as a function of temperature: Hmix(T,10Pa)=0.11205T(K)+0.51594molkJ e) Would more or less energy be required if LPG were a gas at 1 [3] bar? Justify yourStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


