Using the steam tables, estimate the values for the thermal expansion coeffi cient, ,and the isothermal compressibility,
Question:
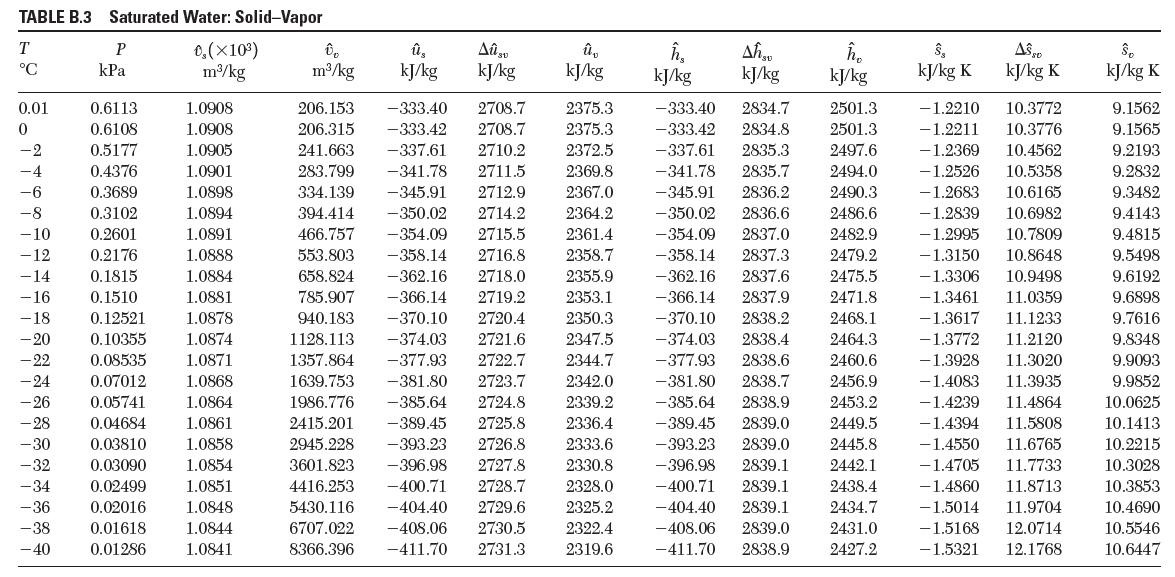
Using the steam tables, estimate the values for the thermal expansion coeffi cient, β,and the isothermal compressibility, k, of liquid water at 20°C and 100°C.
Transcribed Image Text:
Symbols Used in the Steam Tables T P Subscripts 1 S V lv su Temperature Pressure Specific volume Specific internal energy Specific enthalpy Specific entropy C kPa or MPa m/kg kJ/kg kJ/kg kJ/kg K Liquid in equilibrium with vapor Solid in equilibrium with vapor Vapor in equilibrium with liquid or solid Change by evaporation Change by sublimation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
what ways can organizations leverage strategic partnerships, alliances, and ecosystem collaborations to enhance strategic planning outcomes, leverage complementary capabilities, and exploit synergies...
-
Shalit company uses job order cost system and has established a predetermined overhead application rate for the current year of 150% of direct labor cost,based on budgeted overhead of $1800000 and...
-
This question involves using the steam tables in Appendix A, but answer questions A through C before looking at the steam tables. A . The density of liquid water at ambient conditions is about 1 g/cm...
-
Internal Company Assessment (Strategic Analysis Output) on Riise Ev converting Canada post delivery cars to electronic finding out firm strengths weakness liabilities problem constraints...
-
Which of the items in the list of transactions in question A5.1 will have an effect on an income statement (profit and loss account)? (a) Owner puts cash into the business. (b) Buy a vehicle for...
-
A delivery truck travels from point A to point B and back using the same route each clay there are four traffic lights on the route. Let Xi denote the number of red lights the truck encounters going...
-
E12.5. Explaining a Change in Profitability (Medium) Consider the following financial information: Summary Balance Sheets at December 31 2009 2008 2007 Cash $ 100 $ 100 $ 120 Short-term investments...
-
Kleppel et al. (2004) report on a study of wetlands in upstate NewYork. Four wetlands were selected for the study: Two of the wetlands drain watersheds from small towns and the other two drain...
-
nilar to On July 1, 20X6, Steelers Inc. purchased $5,000 of merchandise from Eagles Co. with credit terms of 2/10, n/30. Steelers Inc. paid for the merchandise on July 6, 20X6. What is the amount of...
-
Use the Rackett equation to calculate the liquid-phase molar volume of each of the following species at the same temperature as the measured values reported. Which species had the greatest absolute...
-
Determine expressions for the thermal expansion coeffi cient, , and the isothermal compressibility, k, for an ideal gas.
-
How is it possible that reciprocal dumping can be beneficial for aggregate welfare if identical commodities are moving between countries and transportation costs are being incurred?
-
POTI ENTERPRISES LTD. STATEMENT OF INCOME FOR THE YEAR ENDED DECEMBER 31 (current year) SALES $600,000 COST OF SALES: $50,000 OPENING INVENTORY 250,000 PURCHASES 300,000 CLOSING INVENTORY 60,000...
-
10. Describe a qualified defined contribution plan for the self-employed and discuss the advantages and disadvantages in adopting this type of plan. 11. Describe a SEP IRA and discuss the advantages...
-
7.) In 1999, the average percentage of women who received prenatal care per country is 80.1%. Table #7.3.9 contains the percentage of woman receiving prenatal care in 2009 for a sample of countries...
-
Describe A demographic profile of the population and community that will be served through the reinvented Human Service program. The description must include all eligibility requirements (i.e.,...
-
You work for a major financial institution. Your branch handles customer calls from a wide variety of individuals. Recently, you've noticed an increase in calls from individuals from African...
-
Why might a government want to establish credibility with regard to fighting inflation?
-
Citing a scientific article, explain in your own words, how DNA fingerprinting has been used in forensic science to solve crimes and why it may not always be accurate or effective.
-
Deduce the structure of a compound having the mass spectrum and 1H NMR spectrum presented in Figure 13.43.
-
Figure 13.44 presents several types of spectroscopic data (IR, 1H NMR, 13C NMR, and mass spectra) for a particular compound. What is it?
-
[18]-Annulene exhibits a 1H NMR spectrum that is unusual in that in addition to a peak at ? 8.8 ppm, it contains a second peak having a chemical shift ? of - 1.9 ppm. A negative value for the...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


