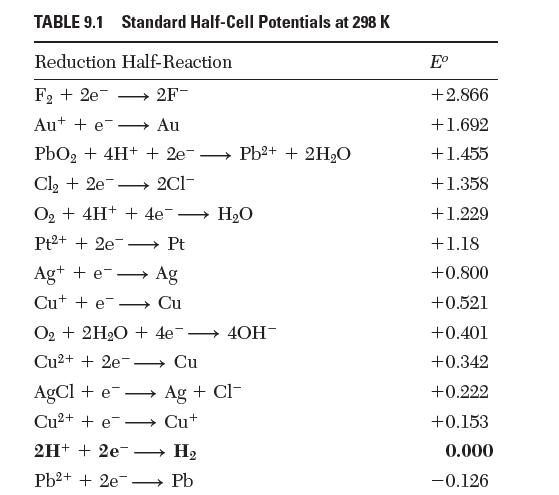
Verify that the standard half-cell potentials reported in Table 9.1 for the reactions between cupric, cuprous, and
Question:
Verify that the standard half-cell potentials reported in Table 9.1 for the reactions between cupric, cuprous, and solid copper![]()
and![]()
Transcribed Image Text:
Cu+e Cut, Cu+ + 2 e Cu,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Verify that the standard matrix of the projection onto W in Example 7.31 (as constructed by Theorem 7.11) does not depend on the choice of basis. Take as a basis for W and repeat the calculations to...
-
"I'm not sure we should lay out $300,000 for that automated welding machine," said Jim Alder, president of the Superior Equipment Company. "That's a lot of money, and it would cost us $84,000 for...
-
Verify that the standard matrix of the projection onto W in Example 7.31 (as constructed by Theorem 7.11) does not depend on the choice of basis. Take as a basis for W and repeat the calculations to...
-
What is the effect of a viscosity (competence) difference between strain markers and the matrix?
-
How much typical storage redundancy do cloud storage providers like Dropbox, iCloud Drive, Google Drive and OneDrive use? In other words, how do cloud storage vendors prevent files from getting lost...
-
A copper wire 100 m long must experience a voltage drop of less than 1.5 V when a current of 2.5 A passes through it. Using the data in Table 18.1, compute the minimum diameter of thewire. Electrical...
-
Generation Ys entitlement mentality. The current workforce is dominated by Generation Ypeople born between 1982 and 1999. These workers have a reputation as having an entitlement mentality (e.g.,...
-
Focusing managerial attention on a single metric may result in undesirable employee behavior and adverse business consequences. For each of the following scenarios, identify the undesirable results...
-
4, Depreciation is usually recorded: a, From the beginning of the accounting year in which an asset is purchased. b, From the actual date of purchase. c, From the first of the month nearest the...
-
Hydrogen-based fuel cells show promise as an alternative fuel source. They use a galvanic cell in which oxygen gas is supplied to one compartment and hydrogen gas to another. The reduction of O2 gas...
-
Electrolysis of NaCl is used to manufacture NaOH, Cl2, and H2. Answer the following questions: (a) Determine the overall reaction and each half-cell reaction. (b) Write the process in terms of...
-
The standard 13C NMR spectrum of phenyl propanoate is shown here. Predict the appearance of the DEPT-90 and DEPT-135 spectra. 13C NMR 0-C-CH2CH3 pheny! propanoate 200 180 160140 10 100 80 40 20 0 8...
-
in a thermodynamics, a phase means what?
-
Give me a 3 python codes in (Discrete Mathematics course) for : 1-basic algorithm 2- the growth of functions 3- Complexity of Algorithms And explain how its works with examples.
-
Using the perpetual inventory system, calculate the ending inventory and Cost of Goods Sold under each of the following methods. Beginning Inventory 10 units @ $1 Purchases January 5 January 20 20...
-
the manager of wongs food express estimates operating costs for the year will total $300,000 for fixed costs. 28. find the sales dollars required with a contribution margin ratio of 40 percent to...
-
Tara Williams and Tilly North had been yoga buddies for almost a decade. Yoga was an escape from the daily stresses of being working parents for both of them. One thing Tara and Tilly always talked...
-
What is parity pricing? How does it differ from other forms of competition-based pricing?
-
Suppose the concentration of glucose inside a cell is 0.1 mm and the cell is suspended in a glucose solution of 0.01 mm. a. What would be the free energy change involved in transporting 10-o mole of...
-
Studies of combustion reactions depend on knowing the concentrations of H atoms and HO radicals. Measurements on a flow system using EPR for the detection of radicals gave information on the...
-
I.D. Chapple-Sokol, Cl Giunta, and R.G. Gordon (J Electrochem Sac 136,2993 (1989)) proposed the following radical chain mechanism for the initial stages of the gas-phase oxidation of silane by...
-
For many years the reaction Hz (g) + I2 (g) →7 2 HI (g) and its reverse were assumed to be elementary bimolecular reactions. However, I.H. Sullivan (J. Chem. Phys. 46, 73 (1967)) suggested that...
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


