What is the composition of vapor that is in equilibrium with a liquid mixture with the following
Question:
What is the composition of vapor that is in equilibrium with a liquid mixture with the following composition at 250 K? What is the pressure? Assume ideal behavior.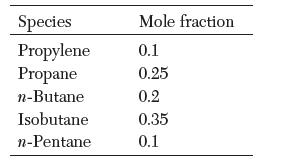
Transcribed Image Text:
Species Propylene Propane n-Butane Isobutane n-Pentane Mole fraction 0.1 0.25 0.2 0.35 0.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The table below shows the vapor pressures, VP (kPa) for nhexane and noctane. (1) Assuming ideal solution and gas behavior, construct Txy and xy diagrams for this system at 101 kPa. (2) When a liquid...
-
A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant pressure of 1 atm (101.3 kPa, 760 tort) from a...
-
What drives competition in commercial aircraft business? Is competition fair in commercial aircraft? As Boeing, what are your options for responding to Airbus? What would you do in 1991?
-
Tell whether the given side lengths of a triangle can represent a right triangle. 36, 48, and 60
-
From the following facts, calculate what Adam Dell must pay Black Co. for the purchase of a bedroom set. Sale terms are 2/10, n/30. a. Invoice price before tax, $5,000, dated April 5. b. Returned one...
-
Does the following statement make sense? The average velocity of the car at 9 a.m. was 60 km/h.
-
Participating in a market. LO.1
-
Kimble and Sanchez, CPAs, offer three types of services to clients: auditing, tax, and small business accounting. Based on experience and projected growth, the following billable hours have been...
-
On January 1, 2016, the Allegheny Corporation purchased machinery for $115,000. The estimated service ble of the machinery is 10 years and the estimated residual value is $5,000. The machine is...
-
A liquid mixture containing 40% cyclohexane, 20% benzene, 25% toluene, and 15% n-heptane is in equilibrium with its vapor at 1 bar. Determine the temperature and the vapor composition.
-
What is the lowest temperature to which a vapor mixture of 1 mole n-pentane and 2 moles n-hexane at 1 bar can be brought without forming liquid? Assume the liquid forms an ideal solution.
-
The payroll register of Tri-State Construction Co. indicates \(\$ 25,650\) of social security withheld and \(\$ 6,750\) of Medicare tax withheld on total salaries of \(\$ 450,000\) for the period....
-
The relevance ( Relevance ) and the reliability ( Reliability ) represent two characters Key qualitative statistics of information n accountant. What What do these two mean? terms in an accounting...
-
A virtual memory system has a page size of 1024 bytes, six virtual pages, and five physical page frames. The page table is shown in Table Q2(d) as follows: Virtual Page Number (VPN) 0 Page Frame...
-
31. z = x + 2xy, determine which of (I)-(II) in Figure 12.31 are cross- sections with x fixed and which are cross-sections with y fixed. (1) (11) -2 -2+
-
WHAT DOES SOCIETY EXPECT FROM ORGANIZATIONS AND MANAGERS? Introduction: TOMS Shoes has a unique idea to promote corporate social responsibility. For each pair of shoes it sells, it donates a pair to...
-
1. A car heading east turns right at a corner. The car turns at a constant speed of 20.0 m/s. After 12 s, the car completes the turn, so that it is heading due south at 20.0 m/s. Calculate the car's...
-
The Fed used broad powers to act as a lender of last resort during the 20072009 financial crises. If there had not been a lender of last resort, what would the effect have been on banks and other...
-
Which should drive action planning more, strengths or weaknesses? That is, is it more important to build on your strengths or to reduce your weaknesses? Explain.
-
On a cold, dry morning after a frost, the temperature was -5C and the partial pressure of water in the atmosphere fell to 0.30 kPa. Will the frost sublime? What partial pressure of water would ensure...
-
Given that Cp = T(V/T)p - V, derive an expression for .u in terms of the van der Waals parameters a and b, and express it in terms of reduced variables. Evaluate u at 25C and 1.0 atm, when the molar...
-
The normal boiling point of hexane is 69.0C. Estimate (a) Its enthalpy of vaporization and (b) Its vapour pressure at 25C and 60C.
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False
-
Which of the following examples of business-use property is NOT eligible for Section 1231 treatment when sold at a gain? * Sale of land held for three years. Net gain from a casualty gain on a dump...

Study smarter with the SolutionInn App


