Question: A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant
A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant pressure of 1 atm (101.3 kPa, 760 tort) from a temperature of 60?C to 90?C. Using the following T-x-y experimental data, perform calculations to determine the answers to parts (a) through (f).
(a) At what temperature does vaporization begin?
(b) What is the composition of the first bubble of equilibrium vapor formed?
(c) What is the composition of the residual liquid when 25 mol% has evaporated? Assume that all vapor formed is retained within the apparatus and that it is completely mixed and in equilibrium with the residual liquid.
(d) Repeat part (c) for 90 mol% vaporized.
(e) Repeat part (d) if, after 25 mol% is vaporized as in part (c), the vapor formed is removed and an additional 35 mol% is vaporized by the same technique used in part (c).
(f) Plot the temperature versus the percent vaporized for parts (c) and (e).
(g) Use the following vapor pressure data in conjunction with Raoult's and Dalton's laws to construct a T-x-y diagram, and compare it for the answers obtained in parts (a) and (f) with those obtained using the experimental T-x-y data. What do you conclude about the applicability of Raoult's law to this binary system?
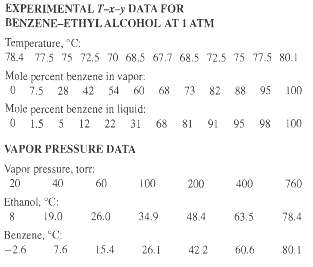
EXPERIMENTAL T-x-y DATA FOR BENZENE-ETHYLALCOHOL AT 1 ATM Temperature, "C: 78.4 77.5 75 72,5 70 68.5 67.7 68.5 72.5 75 77.5 80.1 Mole percent benzene in vapor: 0 7.5 28 42 54 60 Mule percent benzene in liquid: 0 1.5 5 12 68 73 82 88 95 100 68 81 91 95 98 22 31 100 VAPOR PRESSURE DATA Vapor pressure, torr: 20 200 40 60 100 400 760 Ethanol, "C: 19.0 26.0 34.9 48.4 63.5 78.4 Benzene, "C: 60.6 -2.6 7.6 15.4 26.1 422 80.1
Step by Step Solution
3.55 Rating (166 Votes )
There are 3 Steps involved in it
See plot of Txy data on next page as drawn with a spreadsheet Curved instead of straight lines connecting the points would be a good improvement a For a benzene mole fraction of 025 a vertical line fr... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (95).docx
120 KBs Word File


