Question: A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant
A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant pressure of 1 atm from 60C to 90C. Using the following T–x–y experimental data, determine
(a) The temperature where vaporization begins;
(b) The composition of the first bubble of vapor;
(c) The composition of the residual liquid when 25 mol % has evaporated, assuming that all vapor formed is retained in the apparatus and is in equilibrium with the residual liquid.
(d) Repeat part (c) for 90 mol% vaporized.
(e) Repeat part (d) if, after 25 mol% is vaporized as in part (c), the vapor formed is removed and an additional 35 mol% is vaporized by the same technique used in part (c).
(f) Plot temperature versus mol% vaporized for parts (c) and (e).
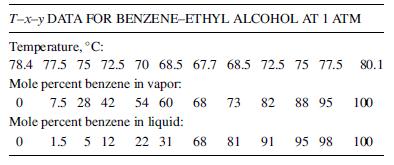
(g) Use the following vapor pressure data with Raoult’s and Dalton’s laws to construct a T–x–y diagram, and compare it to the answers obtained in parts (a) and (f) with those obtained using the experimental T–x–y data. What are your conclusions?
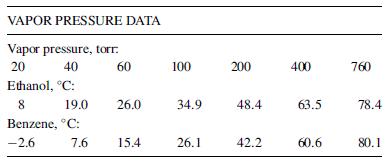
T-x-y DATA FOR BENZENE-ETHYL ALCOHOL AT 1 ATM Temperature, C: 78.4 77.5 75 72.5 70 68.5 67.7 68.5 72.5 75 77.5 80.1 Mole percent benzene in vapor. 0 7.5 28 42 54 60 68 73 Mole percent benzene in liquid: 01.5 5 12 22 31 82 88 95 68 81 91 95 98 100 100
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
a The temperature where vaporization begins is 60C b The composition of the first bubble of vapor is 25 mol benzene and 75 mol ethyl alcohol c The com... View full answer

Get step-by-step solutions from verified subject matter experts


