You wish to determine the enthalpy of vaporizaton of species A. You are able to fi
Question:
You wish to determine the enthalpy of vaporizaton of species A. You are able to fi nd the following data: Species A has a normal boiling point of 207.3 K, and at 20.0 atm, it boils at 301.5 K.
The following equation of state is reported: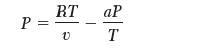
with a = 25 K. As best as you can, estimate Δhvap. State any assumptions that you make.
Transcribed Image Text:
P = RT V aP T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
You wish to determine the sequence of a polypeptide that has the following amino acid composition. (a) What is the maximum number of peptides you can expect if you cleave the polypeptide with...
-
Referring to Problem 14.41 on page 506, you have decided to analyze whether there are differences in fixed acidity, chlorides, and pH between white wines and red wines (0 = white 1 = red). Using the...
-
Determine the enthalpy of combustion of methane (CH4) at 25oC and 1 atm, using the enthalpy of formation data from Table A-26. Assume that the water in the products is in the liquid form. Compare...
-
Name the following compounds.
-
Write a paper on "Using Database Software to Design a Customer System for Auto Sales"
-
Dillon Products manufactures various machined parts to customer specifications. The company uses a job-order costing system and applies overhead cost to jobs on the basis of machine-hours. At the...
-
Define what a methodology is and the role it serves. AppendixLO1
-
A personnel psychologist measures the average tenure of employees. The average number of years 41 employees work before resigning or retiring is 12.2 years, with a GM of 0.365 years. a. What point...
-
As part of an activity-based management (ABM) project, one activity that has been identified by the analyst for elimination is reconciliation. This is a costly accounting control. The analyst is...
-
In Example 6.2, we developed an expression for gd - gn vs. T for the protein lysozyme (l) between its native phase, n, and its denatured phase, d, where unfolding occurs. Develop the same expression...
-
At 1 atm titanium melts at 1941 K and boils at 3560 K. Its triple point pressure is 5.3 Pa. Using only these data, estimate the enthalpy of vaporization of titanium. You will need to think about a...
-
The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2 (~79%) and O2 (~20%). In the cylinder of an automobile engine,...
-
Absorption linewidth for an absorbing atomic transition. Consider the curves of power transmission T(w) = exp[-2am(w)L] through an atomic medium with a lorentzian resonant transition, plotted versus...
-
EXAMPLE 05.04 Z Write the force and the couple in the vector form (with rectangular/Cartesian components). Use C = 180 N-m and P = 500 N O INDIVIDUAL Submission (IS12) D x 400 mm B C 300 mm A 400 mm...
-
1.XYZ Corporation budgets factory overhead cost of P500,000 for the coming year. Compute for the overhead cost applied to the job. The following data are available: Budgeted annual overhead for...
-
OP Technologies Manufacturing manufactures small parts and uses an activity-based costing system. Activity Materials Assembling Packaging Est. Indirect Activity Costs $65,000 $242,000 $90,000...
-
3. Solve Example 3.7 (Bergman, Lavine, Incropera, and DeWitt, 6th Ed., pp. 129-132, or 7th Ed., pp. 145-149, or 8th Ed., pp. 134-138), but use the finite difference method. T T = 30C Insulation-...
-
When might zone pricing be particularly difficult to manage?
-
In the series connection below, what are the respective power consumptions of R, R2, and R3? R R www 4 V=6V P1-3 W; P2=3W; and P3= 3 W OP10.5 W; P2-1 W; and P3= 1.5 W P1=1.5 W; P2=1 W; and P3= 0.5 W...
-
Consider some of the precautions that must be taken when conducting single-molecule spectroscopy experiments. (a) What is the molar concentration of a solution in which there is, on average, one...
-
Suppose that you are a colour chemist and had been asked to intensify the colour of a dye without changing the type of compound, and that the dye in question was a polyene. Would you choose to...
-
Estimate the oscillator strength (see Problem 14.16) of a charge transfer transition modeled as the migration of an electron from an His orbital on one atom to another His orbital on an atom a...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


