You wish to determine the fugacity of a in a liquid mixture with 1 mole of a
Question:
You wish to determine the fugacity of a in a liquid mixture with 1 mole of a and 4 moles of b at 30 kPa and 20°C. At this temperature, the saturation pressure of pure a is 50 kPa. The excess Gibbs energy for a mixture of a and b has been fi t to the following relation: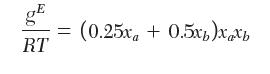
Transcribed Image Text:
gE = (0.25x + 0.5x)x a Xb
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Madhvendra Pandey
Hi! I am Madhvendra, and I am your new friend ready to help you in the field of business, accounting, and finance. I am a College graduate in B.Com, and currently pursuing a Chartered Accountancy course (i.e equivalent to CPA in the USA). I have around 3 years of experience in the field of Financial Accounts, finance and, business studies, thereby looking forward to sharing those experiences in such a way that finds suitable solutions to your query.
Thus, please feel free to contact me regarding the same.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
You wish to determine the sequence of a polypeptide that has the following amino acid composition. (a) What is the maximum number of peptides you can expect if you cleave the polypeptide with...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Suppose the (1) + (2) system exhibits liquid-liquid immiscibility. Suppose we are at a state where G 1 /RT = 0.1 and G 2 /RT = 0.3. The Gibbs energy of mixing quantifies the Gibbs energy of the...
-
The following are the Ledger Balance (in thousands) extracted from the books of Vaishnavi Bank Ltd as on March 31, 2016. The bank's Profit and Loss Account for the year ended and Balance Sheet as at...
-
Wonder Kid Enterprises Company has produced the following results over the last three years: Management has just issued its annual report for 20XZ and a fair reading of their commentary is that the...
-
Draw a figure like Figure 2.1 to represent the following situation. a. A firm starts out with $10 million in cash. b. The rate of interest r is 10 percent. c. To maximize NPV the firm invests today...
-
grasp some of the key questions the marketer needs to address in developing this understanding AppendixLO1
-
Salvania Corporation is considering investing in Farnorth Corporation, but is unsure about what level of ownership should be undertaken. Salvania and Farnorth have the following reported incomes:...
-
Toxaway Company is a merchandiser that segments its business into two divisionsCommercial and Residential. The companys accounting intern was asked to prepare segmented income statements that the...
-
Calculate the activity coeffi cients of a binary mixture of 30 mol% acetone (1) in water (2) at 61.1C using the UNIQUAC activity coeffi cient model. Compare the values to the experimentally measured...
-
Your supervisor has assigned you to obtain parameters A and B for the three-suffi x Margules equation to input in the companys phase equilibrium computer database. The binary mixture of interest is...
-
Consider steady heat conduction in a plane wall whose left surface (node 0) is maintained at 30C while the right surface (node 8) is subjected to a heat flux of 1200 W/m 2 . Express the finite...
-
Childhood leukemia, a hematological malignancy, is the most common form of childhood cancer, representing 29% of cancers in children aged 0 to 14 years in 2018. Imagine that you work in the State...
-
Question 1 Approximating functions using linear functions or higher degree polynomials is a very useful scientific tool! This concept generalizes to Taylor Polynomials, but is most simply illustrated...
-
Find the volume of the solid of revolution formed by rotating the specified region R about the x axis. Volume Formula Suppose f(x) is continuous and f(x) 0 on a x b, and let R be the region under the...
-
As machines get older, the cost of maintaining them tends to increase. Suppose for a particular machine, the rate at which the maintenance cost is increasing is approximated by the function C' (t) =...
-
At Edsel Automotive, the management team is planning to expand one of its plants by adding a new assembly line for sport utility vehicles (SUVs). The cost of setting up the new SUV assembly line is...
-
Use the values for nominal GDP and real GDP given in the following table to calculate the inflation rate during 1930: 1929 5103.6 billion 5977.0 billion 1930 Nominal GDP Real GDP $91.2 billion $892.8...
-
Suppose you won a financial literacy competition and are given FJS10000 to invest, with the condition that investment can be done either in, i) Invest in Unit trust of Fiji or Invest in Fijian...
-
In an experiment to measure the molar mass of a gas, 250 cm3 of the gas was confined in a glass vessel. The pressure was 152 Torr at 298 K and, after correcting for buoyancy effects, the mass of the...
-
A certain sample of a gas has a volume of20.00 dm ' at OCand 1.000 atm. A plot of the experimental data of its volume against the Celsius temperature, , at constant p, gives a straight line of slope...
-
A certain gas obeys the van der Waals equation with a =0.76 m6 Pa mol-2, its volume is found to be 4.00 X 10-4 m3 mol-1 at 288 K and 4.0 MPa. From this information calculate the van der Waals...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


