Your colleague reports that the conversion to n-butane from the gas phase hydrogenation reaction of 1-butene increases
Question:
Your colleague reports that the conversion to n-butane from the gas phase hydrogenation reaction of 1-butene increases as temperature increases: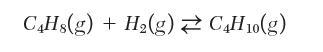
Is this possible? Explain.
Transcribed Image Text:
C4Hs(g) + H(g) C4H0(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
Yes it is possible that the conversion of 1butene to nbutane increases as the temperature increases ...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
Portray in words what transforms you would have to make to your execution to some degree (a) to accomplish this and remark on the benefits and detriments of this thought.You are approached to compose...
-
A baseball player usually has four at bats each game. Suppose the baseball player is a lifetime 0.25 hitter. Find the probability that this player will have: (a) Two hits out of four at bats (b) No...
-
Why is the price elasticity of demand a relative measure? That is, why is elasticity measured in percentage terms rather than absolute terms? 1. So the coeffecient of elasticity will not be dependent...
-
If you move at the speed of light, what shape does the universe take in your frame?
-
An MBAs work-life balance. Many business schools offer courses that assist MBA students with developing good work-life balance habits and most large companies have developed work-life balance...
-
Safety Stocks and Order Points Sache, Inc expects to sell 700 of its designer suits every week. The store is open seven days a week and expects to sell the same number of suits every day. The company...
-
QUESTION 6 (30 Marks) These balances and totals are from Mark and Brake Ltd who manufactures sport equipment Inventory 1 March 20x7 Raw material 14,400 9,500 Work in progress (WIP) Finished goods...
-
Which of the following conditions would you use if you needed to develop an industrial process to produce ethanol from acetylene? Explain. (a) 25C and 1 bar. (b) 250C and 1 bar. (c) 25C and 150 bar....
-
Consider the gas phase hydrogenation reaction of propylene to form propane: To increase the equilibrium conversion, would it help to (a) Increase the pressure? (b) Increase the temperature? (c) Add...
-
1. The text refers to balancing its need to control the costs of recruiting with the need for a given sized workforce. How does Texas Instruments balance those two needs? 2. What are some of the...
-
Business Solutions's second-quarter 2022 fixed budget performance report for its computer furniture operations follows. The $175,750 budgeted expenses include $126,000 in variable expenses for desks...
-
Problem 2 (Numerical Integration) Using switch Statement and functions, write a single code to compute the following integral. 0 10 x +4 dx case 1: RECTANGULAR () // Rectangular rule case 2:...
-
Do you believe the elasticity of illicit narcotics is inelastic and if legalized demand will not increase? Do you also believe that many of society's social ills associated with drugs will ease not...
-
Stockstone Limited makes electric kettles that they currently sell at 13 each. The management believes that the company's equipment could currently produce up to 70,000 units of electric kettles per...
-
Jane Smith has worked for the Widgets, Weezles, and Warblers Corporation for the past 25 years. At a recent "Town Hall" meeting, Jane asked two members of the executive leadership team about their...
-
For each of the following cases, determine the amount of capital gain or loss to report in each year (after taking into account any applicable carry backs) and the capital loss carry forward to 2014,...
-
Refer to the data for problem 13-36 regarding Long Beach Pharmaceutical Company. Required: Compute each division's residual income for the year under each of the following assumptions about the...
-
Name the functional group(s) present in each of the compounds in Problem 2.17.
-
Name the functional group(s) present in each of the compounds in Problem 2.18.
-
Determine whether these structures represent the same compound orisomers: a) b) @ Y il Q0 CHCH3 So d) OCH,
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


