Which of the following conditions would you use if you needed to develop an industrial process to
Question:
Which of the following conditions would you use if you needed to develop an industrial process to produce ethanol from acetylene? Explain.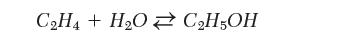
(a) 25°C and 1 bar.
(b) 250°C and 1 bar.
(c) 25°C and 150 bar.
(d) 250°C and 150 bar.
Transcribed Image Text:
CH4+ HO CH5OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Acetylene C2H2 is a different molecule than ethylen...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
If Figure 1 shows our production possibilities frontier during the Great Depression, at which point were we operating? a) point A b) point B c) point C d) point D A Figure 1 B C D
-
Darling Corporation issued 200,000 shares of $20 par value, 5% preferred stock on January 1, 2019, for $4,500,000. In December 2021, Darling declared its first dividend of $800,000. Part 1 Prepare...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Consider a group of 12 employees of whom five are in management and seven do clerical work. Select at random a sample of size 4. What is the probability that there will be one manager in this sample?
-
1. Why might federal spending on roads, waterways, or national security be less subject to direct expenditure offsets than spending on health care or education? 2. What might account for the fact...
-
A long cart moves at relativistic speed v. Sand is dropped into the cart at a rate dm/dt = in the ground frame. Assume that you stand on the ground next to where the sand falls in, and you push on...
-
Thickness of dust on solar cells. The performance of a solar cell can deteriorate when atmospheric dust accumulates on the solar panel surface. In the International Journal of Energy and...
-
Assume that you are working with the results from Problems 11.29 and 11.30. a. What is the value of the test statistic for the interaction effect? b. What is the value of the test statistic for the...
-
Plaza, Inc., acqures 80 percent of the outstanding common stock of Stanford Corporation on January 1, 2018, in exchange for $892,000 cash. At the acquisition date, Stanford's total fair value,...
-
Consider the following liquid phase reactions: A plot of the concentrations in [mol/L], of A, B, and C in a constant-volume batch reactor operating isothermally at 500 K is shown in the following fi...
-
Your colleague reports that the conversion to n-butane from the gas phase hydrogenation reaction of 1-butene increases as temperature increases: Is this possible? Explain. C4Hs(g) + H(g) C4H0(g)
-
On January 1, 2010, Valuation Allowance for Available-for-Sale Securities has a debit balance of $1,500. On December 31, 2010, the cost of the available-for-sale securities was $67,500, and the fair...
-
Global Operations Management is supported by Strategic Supply Chain Management in many ways. Elucidate the following; List and briefly define/describe the Five (5) Components of Strategic Supply...
-
The Alpine House, Inc. is a large winter sports equipment broker. Below is an income statement for the company's ski department for a recent quarter. LA CASA ALPINA, INC. Income Statement - Ski...
-
Two investment portfolios are shown. Investment Portfolio 1 Portfolio 2 ROR Savings Account $1,425 $4,500 2.80% Government Bond $1,380 $3,600 1.55% Preferred Stock $3,400 $2,150 11.70% Common Stock...
-
The following information pertains to JAE Corporation at January 1, Year 1: Common stock, $8 par, 11,000 shares authorized, 2,200 shares issued and outstanding Paid-in capital in excess of par,...
-
Group dynamics are important elements within the leading facet of the P-O-L-C framework. Discuss a time in your professional, school, or personal life when you experienced the Five Stages of Group...
-
Determine the deductible charitable contribution in each of the following instances. a. Charitable contribution of $4,000 and taxable income before charitable contribution of $50,000. b. Charitable...
-
A firm has the following balance sheet: Assets Cash Accounts receivable Inventory Plant and equipment $ 15,000 150,000 92,000 170,000 $427,000 Liabilities and Equity Accounts payable Long-term debt...
-
Which functional group is present in each of thesecompounds? a) CHCHCHCHOCH, ) CHCH,CH,CO,H Careful: how are the O's bonded to the C? e) CHCOCH, b) d) f) OH 0 CNH, H
-
Convert the following structures to skeletal structures: OH a) CHCH,CH,CHCH,CH, b) HC d) || HC OH CH CH HC. H CH3 CH CH H H H H CH f) CHCHCH-CHC-CH CH CH 0 11 c) CH3CCHCHCHCI || C CH3 e) CHC-CHCHCHCH...
-
Convert the following shorthand representations to structures showing all of the atoms, bonds, and unshared electron pairs: d) COOH COH b) OH de c) f) H CN Ni
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...

Study smarter with the SolutionInn App


