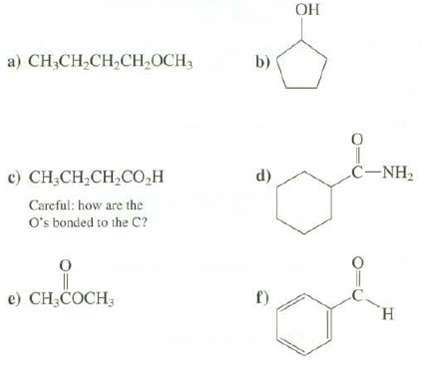
Which functional group is present in each of thesecompounds? a) CHCHCHCHOCH, ) CHCH,CH,CO,H Careful: how are the
Question:
Which functional group is present in each of thesecompounds?
Transcribed Image Text:
a) CH₂CH₂CH₂CH₂OCH, ¢) CHÍCH,CH,CO,H Careful: how are the O's bonded to the C? e) CH₂COCH, b) d) f) OH 0 CNH, H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a Ether b Alcoh...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which transition metal atom is present in each of the following biologically important molecules: (a) Hemoglobin (b) Chlorophylls (c) Siderophores.
-
Name the functional group(s) present in each of the compounds in Problem 2.17.
-
Name the functional group(s) present in each of the compounds in Problem 2.18.
-
Discuss why you would or would not like to work in an organization like this?
-
Explain the purpose of a strategy map and how it relates to a balanced scorecard?
-
Dennis sells short 100 shares of ARC stock at $20 per share on January 15, 2016. He buys 200 shares of ARC stock on April 1, 2016, at $25 per share. On May 2, 2016, he closes the short sale by...
-
COVID-19 control charts. To help deal with the COVID-19 pandemic of 2020, the Montgomery County (PA) Fire & Rescue Service created quality control charts for various virus metrics such as daily call...
-
Calculating Taxes The Locker Co. had $273,000 in taxable income. Using the rates from Table 2.3 in the chapter, calculate the companys income taxes. What is the average tax rate? What is the marginal...
-
8. Hardford Company presents the following data for 2018. Net Sales Cost of Goods Sold Gross receivables (ending balance) $ 2.360.108 $ 1,580,360 S 266.700 The days' sales in receivables (rounded)...
-
Place-Plus, a real estate development firm, is considering several alternative development projects. These include building and leasing an office park, purchasing a parcel of land and building an...
-
Which of these compounds would you expect to be more soluble in water? Explain? CHCHCHCHCOH or CH3CHCHCHCHCOH
-
Convert the following structures to skeletal structures: OH a) CHCH,CH,CHCH,CH, b) HC d) || HC OH CH CH HC. H CH3 CH CH H H H H CH f) CHCHCH-CHC-CH CH CH 0 11 c) CH3CCHCHCHCI || C CH3 e) CHC-CHCHCHCH...
-
Suppose is a probability density function for the random variable X with mean m. Show that its variance satisfies Var (X) = 20 X2f(X ) dX = 2. -
-
The current rate of interest on S-T Treasury Bills = 10%, intermediate term Gov. Bonds = 11%, Lt- Gov. Bonds = 12%, AA rate Corp. Bonds = 13.5% and the rate of inflation is 5%. Holding-period returns...
-
Prepare Income Statement(absorption costing) for the second, third and fourth month. SALES (SP X unit sold) INCOME STATEMENT FORMAT (ABSORPTION COSTING) XXX Less: Cost of Goodsold VARIABLE COST (VC...
-
The following shows the distribution of final exam scores in a large introductory psychology class. The proportion under the curve is given for two segments (short answers-no calculations required)....
-
How much overhead was included in the cost of Job #461 at the beginning of January? * (1 Point). BREAD Co. uses a job order costing system. At the beginning of January, the company had 2 jobs in...
-
3. (3pt.) A state of a physical system is just a description of the system at an instant in time in terms of its properties. In classical mechanics, states are represented by points (in phase space)....
-
The custom of requiring employees to pool or share their tips is illegal, and management should avoid this practice at all times. L01 A. True B. False
-
In a paragraph of approximately 150-200 words, analyze a film or TV/Streaming Show poster of your choosing by focusing on the ways in which representations in the poster are gendered. Include an...
-
Find 1 + x 2 x 5 dx.
-
Propose a mechanism to account for the following reaction: C CH2CI AICI3
-
In the Gatterman-Koch reaction, a formyl group (?CHO) is introduced directly onto a benzene ring. For example, reaction of toluene with CO and HCl in the presence of mixed CuCl/AlCl 3 gives...
-
Treatment of p-tert-butyl phenol with a strong acid such as H2SO4 yields phenol and 2-methyipropene. Propose a mechanism.
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...
-
*Prepare the plant assets section of Amphonie's balance sheet at December 31, 2021 using the information below. At December 31, 2020, Amphonie Company reported the following as plant assets. Land $...

Study smarter with the SolutionInn App


