Question: Which of these compounds would you expect to be more soluble in water? Explain? CHCHCHCHCOH or CH3CHCHCHCHCOH
Which of these compounds would you expect to be more soluble in water? Explain?
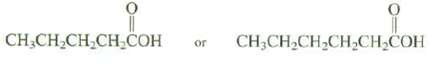
CHCHCHCHCOH or CH3CHCHCHCHCOH
Step by Step Solution
★★★★★
3.29 Rating (164 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Both compounds have a polar hydrophilic part and a nonpolar hydroph... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
15-C-O-O-C (15).docx
120 KBs Word File


