Your coworker has scribbled down the saturation pressures for a pure species from the solid (sublimation) and
Question:
Your coworker has scribbled down the saturation pressures for a pure species from the solid (sublimation) and liquid (evaporation) as follows: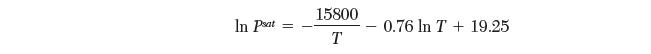
and,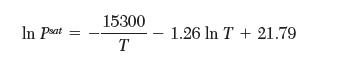
However, in his haste, he forgot to note which equation was for sublimation and which was for evaporation. Please help your coworker by determining the correct matches. Explain your reasoning.
Transcribed Image Text:
In Pat 15800 T 0.76 In T+ 19.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Northern Virginia Community College HOW MUCH FINANCIAL RISK SHOULD YOU TAKE? Mark D. D'Antonio Nova Southeastern University FORT LAUDERDALE, FLORIDA, U.S.A. Abstract A successful retirement...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Following procedures similar to those for the homogeneous problem (see Section 8.4.1), develop the following stress field for a pressurized hole in an infinite nonhomogeneous medium with moduli...
-
Regarding government budgets, explain the incrementalism model (championed by Lindblom). How does it change with the level of government? How is it affected by a divided government? Is it an...
-
Gold Star Rice, Ltd., of Thailand exports Thai rice throughout Asia. The company grows three varieties of riceFragrant, White, and Loonzain. (The currency in Thailand is the baht. which is denoted by...
-
What is a Project Board? What is its role in a PRINCE2 project? AppendixLO1
-
Lanza Research Inc. manufactures high-quality hair care products in California. Copyrighted labels are attached to all products and packaging. In the United States, Lanza sells exclusively to...
-
Epperson Co. had 100,000 common shares outstanding at the beginning of 20x5. On July 1, 20x5, it issued 6% bonds at face value of $500,000. The bonds were convertible into 20, 000 common shares....
-
(a) A pure fl uid shows the following s vs. T behavior. Draw schematically how the chemical potential would change with temperature. (b) A pure substance shows the following v vs. P behavior at...
-
A well-insulated tank with a valve at the top contains saturated water at 5 MPa. The quality of the water is 0.1. (a) What is the ratio of the liquid volume to the vapor volume? (b) The valve is...
-
In Exercises, find all points (x, y) where f (x, y) has a possible relative maximum or minimum. f (x, y) = x 3 + 3x 2 + 3y 2 - 6y + 7
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
Explain the concept of expected revenue. Describe how historical data on full-price demand can be used to calculate expected revenues.
-
Prepare a stock card using the following information A company is registered for GST which it pays quarterly, assume GST was last paid on the 30th of June 2019. It uses weighted average cost...
-
Treat carbon monoxide as a perfect gas and apply equilibrium statistical thermodynamics to the study of its properties, as specified below, in the temperature range 100-1000 K at 1 bar. V = 2169.8...
-
The exchange of deuterium between acid and water is an important type of equilibrium, and we can examine it using spectroscopic data on the molecules. Calculate the equilibrium constant at (a) 298 K...
-
Suppose that an intermolecular potential has a hard-sphere core of radius 'I and a shallow attractive well of uniform depth E out to a distance '2' Show, by using eqn 17.42 and the condition E kT,...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...
-
BE13.2 (LO 1), AP An inexperienced accountant for Silva Corporation showed the following in the income statement: net income \$337,500 and unrealized gain on availablefor-sale securities (before...

Study smarter with the SolutionInn App


