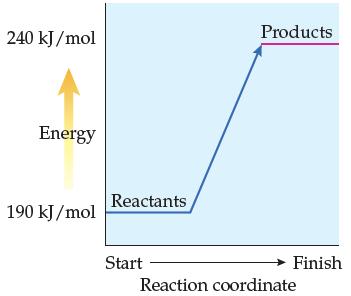
According to the reaction-energy profile in Problem 13.44, the products are higher in energy than the reactants.
Question:
According to the reaction-energy profile in Problem 13.44, the products are higher in energy than the reactants. What must occur during the course of the reaction for this to occur?
Data from Problem 13.44
Judging from the following reaction-energy profile, is the reaction endothermic or exothermic? What is the value of ΔErxn?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





