Assign an oxidation state to each atom in the amino acid glycine: - H-N-C-C T
Question:
Assign an oxidation state to each atom in the amino acid glycine:
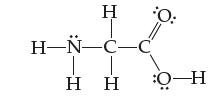
Transcribed Image Text:
Η Η- H-N-C-C T Η Η O-H 0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The following table shows the oxidation states of each atom in the amino acid glycine Atom Oxidation state H 1 C carbonyl carbon 1 C alpha carbon 1 N ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
In carbon dioxide, CO 2 , (a) Assign an oxidation state to each atom in the molecule. (b) How many electrons does the C atom own by oxidation-state electron bookkeeping? (c) How many more or fewer...
-
In chloroform, CHCl 3 , (a) Assign an oxidation state to each atom in the molecule. (b) How many electrons does the C atom own by oxidation-state electron bookkeeping? (c) How many more or fewer...
-
In methane, CH 4 , (a) Assign an oxidation state to each atom in the molecule. (b) How many electrons does the C atom own by oxidation-state electron bookkeeping? (c) How many more or fewer valence...
-
Problem 5-47 Amortizing Loans and Inflation (LO3) Suppose you take out a $108,000, 20-year mortgage loan to buy a condo. The interest rate on the loan is 5%. To keep things simple, we will assume you...
-
The comparative balance sheet of Putnam Cycle Co. at December 31, 2010 and 2009, is as follows: The noncurrent asset, noncurrent liability, and stockholders equity accounts for 2010 are as follows:...
-
For each pair of triglycerides, identify the one that is expected to have the higher melting point. Consult Table 26.1 to determine which fatty acid residues are present in each triglyceride. (a)...
-
. Why are user stories and system features critical components of an effective IT software development process? This isnt what I need! objected the admissions officer at Northwest Regional Hospital....
-
The following data were extracted from the income statement of Brecca Systems Inc.: (a) Determine for each year(1) The inventory turnover and(2) The number of days' sales in inventory. Round to...
-
Manufacturing Dairy Products Co. engaged Nabali & Fares Construction Co. to design and construct a complete modernization of Pinar's manufacturing facility. Construction was begun on February 1, 2018...
-
Identify the oxidizing agent and reducing agent in the reaction IO + 71 + 8 H412 + 4 HO
-
When you turn on an electrical appliance, are you consuming electrons?
-
The researchers from Exercise 2 want to test if the proportions of satisfied employees are the same at for-profit companies as at not-for-profit companies. In Exercise 2 Data collected from 422...
-
Which of the five hazardous attitudes do you display most frequently? What can you do to minimize the presence and impact of these attitudes in your life?
-
What brought you to this course? How do you define Black or Blackness? What do you hope to get out of this class? When you think of Black Culture, what is the first thing that comes to mind? [For...
-
1. What is XBRL Taxonomy? How do you as a preparer of financial statement use the XBRL Taxonomy in locating a label for a specific financial element? 2. What are the benefits of adopting XBRL from...
-
What a business can do to protect and minimize the invasion of privacy for their customers? Think of your experience when visiting a website. What do most websites have you agree to before you do...
-
Based on your interest, skill set, or goals what do you typically contribute when working in groups? What do you need others to contribute due to your lack of interest, skill set, or goals? How do...
-
Let L: V IV be a linear transformation, and let T be a subspace of W. The inverse image of T denoted L-l(T), is defined by L-1(T) = {v V\L(v) T} Show that L-1(T) is a subspace of V
-
A company produces earbuds. The revenue from the sale of x units of these earbuds is R = 8x. The cost to produce x units of earbuds is C = 3x + 1500. In what interval will the company at least break...
-
A current source in a linear circuit has i s = 15 cos (25 t + 25) A (a) What is the amplitude of the current? (b) What is the angular frequency? (c) Find the frequency of the current. (d) Calculate...
-
Given v 1 = 45 sin(t + 30) V and v 2 = 50 cos(t 30) V, determine the phase angle between the two sinusoids and which one lags the other.
-
Transform the following sinusoids to phasors: (a) 20 cos(4t + 135) (b) 8 sin(20t + 30) (c) 20 cos (2t) + 15 sin (2t)
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


