Balance this nuclear reaction and predict the product: 21Pb? + je 0 82- B particle.
Question:
Balance this nuclear reaction and predict the product:
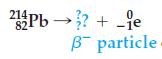
Transcribed Image Text:
21Pb→? + je 0 82- B particle.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
214...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Balance this nuclear reaction and predict the product: 20 Po? + He 209p 84 a particle
-
What would the answer to WorkPatch 16.6 be if there were another beta emission? WorkPatch 16.6 Balance this nuclear reaction and predict the product: 2Pb? + -e 214 82 B particle
-
Show the mechanism of this reaction and predict the stereochemical outcome of this reaction. Me Me Me Me PHNCO * * NO2 only one isomer A. Kozikowski & Co-workers, Tetrahedron Lett. 1982, 23, 2081.
-
What is meant by budgetary control?
-
Are all fixed costs unavoidable?
-
If two electrons are each 1.50 x 10 -10 m from a proton, as shown in Fig. E 21.37, find the magnitude and direction of the net electric force they will exert on the proton. Figure E21.37 65.0
-
In the model of Exercise 8.1, calculate the unique risk-neutral probability for any given horizon T < , and show that the risk-neutral probability of any path depends on t and the parameters Rf , k,...
-
The current assets and current liabilities sections of the statement of financial position of Agincourt Corp. are as follows: The following errors have been discovered in the corporations accounting:...
-
Please answer all questions, I promise to like the answer! Fact Pattern 12-1 A-One Construction Company enters into a contract with Ben to remodel Carol's Home Store, using products from Delta...
-
Suppose 35 14 Si undergoes beta emission. Write a nuclear reaction for this spontaneous change.
-
When a nucleus emits a beta particle: (a) The atomic number increases by 1. (b) The atomic number decreases by 1. (c) The atom changes elemental identity equal to one step to the left in the periodic...
-
Explain the components of Roethlisbergers change model.
-
Solve X+1U6x-13x+2-4x+5
-
Summarize the selected poster's design format, such as the color, layout, font style, size, space, and the subject's analysis format. Also, analyze how the study started. Such as background and...
-
Income statement Prior year Current year Revenues 782.6 900.0 Cost of sales Selling costs Depreciation (27.0) (31.3) Operating profit 90.4 85.7 Interest Earnings before taxes 85.4 78.2 Taxes (31.1)...
-
View the video at the slide title "Lab: Social Media Post" at time 28:20. Link:...
-
Write a program ranges.py in three parts. (Test after each added part.) This problem is not a graphics program. It is just a regular text program to illustrate your understanding of ranges and loops....
-
Which of the following ethers would be obtained in greatest yield directly from alcohols? CH1 CH3OCCH3 CH3 CH,, CH CH20CH2CH2CH3
-
On October 31 Juanita Ortega, owner of Outback Guide Service, received a bank statement dated October 30. Juanita found the following: 1. The checkbook has a balance of $2,551.34. 2. The bank...
-
Find the Fourier transform of the waveform shown in Fig. 18.29 . g(t) -1 1
-
Using Fig. 18.27 , design a problem to help other students better understand the Fourier transform given a wave shape. What is the Fourier transform of the triangular pulse in Fig. 18.27? f(t) f(0) t1
-
Design a problem to help other students better understand how to find the exponential Fourier series of a given periodic function. Given the periodic function f(t) = t 2 , 0 < t < T obtain the...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


