Both diamond and graphite are network solids consist solely of carbon atoms: In diamond, every carbon atom
Question:
Both diamond and graphite are network solids consist solely of carbon atoms:
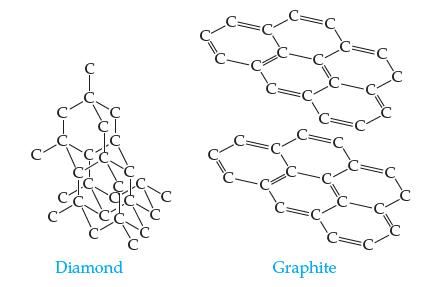
In diamond, every carbon atom is bonded to four other carbon atoms. In graphite, every carbon atom is bonded to three other carbon atoms, creating sheets of atoms that lie on top of one another.
(a) Which type of intermolecular forces exist between the sheets of carbon atoms in graphite?
(b) Diamond is an extremely hard substance, whereas graphite is a soft, slippery substance often used as a lubricant. Use intramolecular and intermolecular forces to explain this difference in physical properties.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





