Cisplatin is a molecule with a platinum (Pt) atom in the middle that is bound to two
Question:
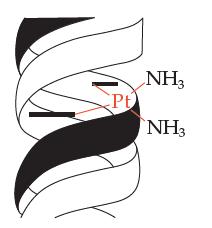
Cisplatin is a molecule with a platinum (Pt) atom in the middle that is bound to two chlorine atoms and two ammonia atoms (NH3). It is an effective anticancer drug (chemo) because in the body the two chlorines fall off and the Pt atom binds to the two strands of the DNA double helix of malignant tumor cells. Therefore, the DNA cannot split apart and replicate, which keeps the tumor cells from growing.
Notice that the bonding geometry about the Pt atom is flat (a square), which projects the ammonia atoms away from the DNA strands and allows the Pt to get close enough to bind the DNA strands. What do you think would happen if the geometry about the Pt atom were not flat such that the ammonia atoms were more pointed toward the DNA strands?
Step by Step Answer:

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver





