Consider a mercury manometer, shown below When the trapped gas pushes the mercury level down in the
Question:
Consider a mercury manometer, shown below
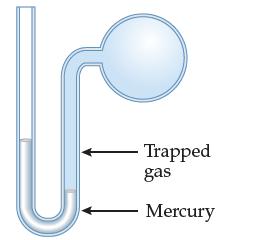
When the trapped gas pushes the mercury level down in the right tube, it causes the mercury to climb to a greater height in the left tube. It will always be true that the amount the mercury column goes down on the right is exactly the same as the increase in the height on the left. In other words, the changes in the column heights always sum to zero. How does this situation compare to adjusting the H3O+ and OH– concentrations in an aqueous solution?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





