Consider the following unbalanced combustion reaction: (a) Balance the equation. (b) How many moles of CO 2
Question:
Consider the following unbalanced combustion reaction:
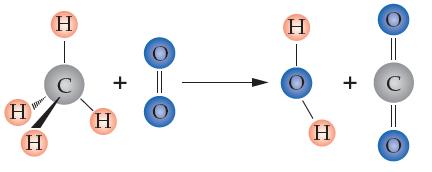
(a) Balance the equation.
(b) How many moles of CO2 will be generated from using excess methane and 5.0 moles of O2?
(c) How many molecules of CO2 will be produced from using excess methane and 5.0 g of O2?
Transcribed Image Text:
Η Η Η C Η H Η + C =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The balanced equation is CH4 2O2 CO2 2H2O b 50 moles of ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The reaction between potassium superoxide, KO2, and CO2, 4 KO2 + 2 CO2 2K2CO3 + 3 O2 is used as a source of O2 and absorber of CO2 in self-contained breathing equipment used by rescue workers. (a)...
-
The complete combustion of octane, C8H18, the main component of gasoline, proceeds as follows: 2 C8H18(l) + 25 O2(g) 16 CO2(g) + 18 H2O(g) (a) How many moles of O2 are needed to burn 1.50 mol of...
-
2.26. The engine-valve system of Fig. P2.26 consists of a rocker arm of moment of inertia J.a valve of mass m and a spring spring of mass m. Determine its effective mass at A. ms m, FIGURE P2.26....
-
A sealed flask contains water and oxygen gas at 25C. The O 2 gas has a partial pressure of 1.5 atm. (a) What is the concentration of O 2 in the water? (b) If the pressure of O 2 in the flask is...
-
The numbers below are for Iffy Company and Model Company for the year 2011: 1. Compute return on equity, return on sales, asset turnover, and the assets-to-equity ratio for both Iffy and Model. 2....
-
Pitt Enterprises manufactures jeans. All materials are introduced at the beginning of the manufacturing process in the Cutting Department. Conversion costs are incurred uniformly throughout the...
-
1 Identify three reasons why the counsellor failed to adjust to living and working in the Middle East. Rank these reasons in order of importance.
-
The following pertains to the Cereal Division of McKenzie Corporation. Conversion costs for this division were 80 percent complete as to beginning work-in-process inventory and 50 percent complete as...
-
Fanning Manufacturing Company reported the following data regarding a product it manufactures and sells. The sales price is $48. Variable costs Manufacturing $ 18 per unit Selling 4 per unit Fixed...
-
When 490.0 mg of iron reacts with excess bromine, a mixture of FeBr 2 and FeBr 3 is produced. It is determined that 35.5% of the mixture is iron(II) (bromide). What is the total mass of FeBr 2 /FeBr...
-
The bicycle shown below has a formula of frame 1 tires 2 and weighs 88.0 pounds (the frame includes the pedals, chain, and seat). (a) Given that the mass percent of the frame is 75.0%, how much does...
-
Which statement best describes this class? A. It can be serialized. B. It is well encapsulated. C. It is immutable. D. It is both well encapsulated and immutable. E. None of the above as the code...
-
Construct a 90% confidence interval for the population standard deviation o at Bank B. Bank B 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Jamila Traders has a head office in Nanyuki and an autonomous branch in Thika. The trial balances of the head office and the branch as at 30 September 2014 were as follows: Head office Sh. Sh. Thika...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
ROI analysis using the DuPont model a. Firm A has a margin of 7%, sales of $980,000, and ROI of 19.6%. Calculate the firm's average total assets. b. Firm B has net income of $259,200, turnover of...
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
A particle of rest mass m has three-velocity v. Find its energy correct to terms of order |v|4. At what speed |v| does the absolute value of 0(|v|4) term equal of the kinetic-energy term 1/2m|v|2?
-
CdF2 (s) Cd+ (aq) + 2 F- (aq) 1. A saturated solution of CdF2 is prepared. The equilibrium in the solution is represented above. In the solution [Cd+] eq = 0.0585 M and [F-] eq = 0.117 M. a....
-
Draw all stereoisomers of l-isoleucine. In each stereoisomer, assign the configuration (R or S) of all chirality centers.
-
Arginine is the most basic of the 20 naturally occurring amino acids. At physiological pH, the side chain of arginine is protonated. Identify which nitrogen atom in the side chain is protonated.
-
Histidine possesses a basic side chain which is protonated at physiological pH. Identify which nitrogen atom in the side chain is protonated.
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


