Consider the two nonpolar hydrocarbon molecules, both of formula C 5 H 12 . Having exactly the
Question:
Consider the two nonpolar hydrocarbon molecules, both of formula C5H12.
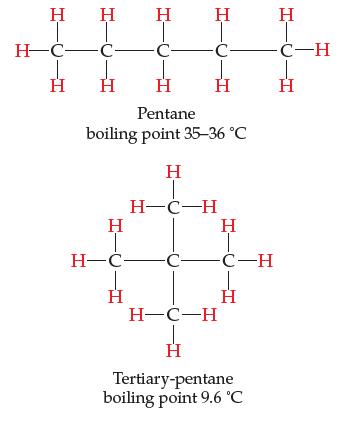
Having exactly the same formula, they have exactly the same number of electrons, yet pentane has a higher boiling point than tertiary-pentane. Discuss why and come up with a theory that explains why.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





