Consider the two square-planar molecules shown below. Which would you expect to have a higher boiling point,
Question:
Consider the two square-planar molecules shown below. Which would you expect to have a higher boiling point, and why?
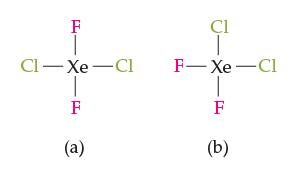
Transcribed Image Text:
F CI-Xe Cl 1-8 F (a) Cl F-Xe-Cl T F (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
Between the two squareplanar molecules shown a FClXeClF b FXeClF We can predict that molecule a FClX...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
a. Which compound would you expect to have a higher dipole moment, methyl acetate or butanone? b. Which would you expect to have a higher boiling point? CH3COCH methyl acetate CH,CCH,CH butanone
-
What is the shape factor of the shape below? A. 0.96 B. 1.22 C. 1.04 D. 2.11 E. 17.8 1 cm 1 cm 3 cm 1 cm 3 cm 1 cm 1 cm 1 cm
-
The following compounds have the same molecular formulas (C4H10). Which one would you expect to have a higher boiling point?
-
Write the complete APT part program to profile mill the outside edges of the part. The part is 15 mm thick. Tooling = 30 mm diameter end mill with four teeth, cutting speed = 150 mm/min, and feed =...
-
Describe how diversity of workers has been impacting organizations, including organizations for which you have worked recently.
-
Based on the facts and results of Problems 41-47, provide the income tax footnote rate reconciliation for Kantner.
-
Why is BIRCH appropriate for streaming data?
-
The accounts in the ledger of Leaf Co. as of December 31, 2014, are listed in alphabetical order as follows. All accounts have normal balances. The balance of the cash account has been intentionally...
-
During its most recent fiscal year, Raphael Enterprises sold 200,000 electric screwdrivers at a price of $15.00 each. Fixed costs amounted to $400,000 and pretax income was $600,000. What amount...
-
CO 2 is a linear molecule, but just suppose it was possible to do the following (see below). If you could, what would you predict would happen to its melting point? Explain. 0=C=0 0-
-
What is wrong with the following diagram when it comes to explaining London forces?
-
Describe how the following techniques are used in the study of chemical dynamics: infrared chemiluminescence, laser-induced fluorescence, multiphoton ionization, resonant multiphoton ionization,...
-
In Exercises 25-28, construct a data set that has the given statistics. N = 8 2 3
-
Sample SAT scores for eight males and eight females are listed. Males 1010 1170 1410 920 1320 1100 690 1140 Females 1190 1010 1000 1300 1470 1250 840 1060
-
Best Actor 2018: Gary Oldman, Age: 59 Best Supporting Actor 2018: Sam Rockwell, Age: 49 The table shows population statistics for the ages of Best Actor and Best Supporting Actor winners at the...
-
Consider a market dominated by just two airlines, American and United. Each can choose to restrict capacity and charge a high price or expand capacity and charge a low price. If one of the two...
-
Using the product structure for Alpha in Solved Problem 14.1, and the following lead times, quantity on hand, and master production schedule, prepare a net MRP table for Alphas. Data From Problem...
-
Find the area of the surface generated by revolving the curve x = t + 7, y = t2/2 + 7, for - 7 t 7 about the y-axis.
-
Using Gauss-Jordan elimination, invert this matrix ONLY 0 0 0 0 1
-
What is the expression for the diffusion coefficient, D, in terms of gas kinetic theory parameters? How is D expected to vary with an increase in molecular mass or collisional cross section?
-
What is the general relationship between the spatial gradient in a system property and the flux of that property?
-
What is Brownian motion?
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


