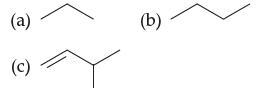
Convert each line drawing to a structural formula, then give the molecular formula and describe the hydrocarbon
Question:
Convert each line drawing to a structural formula, then give the molecular formula and describe the hydrocarbon as being linear or branched:
Transcribed Image Text:
(a) (c) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Propane Lineangle formula a Butane Lineangle formula Linear c 3Methyl1but...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Which molecule in Problem 17.39 is unsaturated? Data from Problem 17.39 Convert each line drawing to a structural formula, then give the molecular formula and describe the hydrocarbon as being linear...
-
Describe in words how you would do each of the following preparations. Then give the molecular equation for each preparation. a. CuCl2(s) from CuSO4(s) b. Ca(C2H3O2)2(s) from CaCO3(s) c. NaNO3(s)...
-
Describe in words how you would do each of the following preparations. Then give the molecular equation for each preparation. a. MgCl2(s) from MgCO3(s) b. NaNO3(s) from NaCl(s) c. Al(OH)3(s) from...
-
Which of the following statements are true about REST? Pick ONE OR MORE options Logical URLs should be used instead of physical URLS Adal URLs must always be used in REST response A paging technique...
-
A consumer is in equilibrium at point A in the accompanying figure. The price of good X is $5.a. What is the price of good Y?b. What is the consumer??s income?c. At point A, how many units of good X...
-
You have a solution that contains Ca 2+ ions and another that contains Na + ions. How would adding a solution that contains CO 2- 3 ionss enable you to tell which is which?
-
While reconciling costing profits with financial profits, under recovery of works overheads is (a) Deducted (b) Added (c) Not included (d) Doubled
-
Microtech Incorporated has decided to package its cell phone in a smaller, recyclable package. Additionally, the company will discontinue the practice of shipping each phone with a 250-page user...
-
The adjusted trial balance for Kylo Ren's Lightsaber Company at December 31, 2021, is presented below: Credit Debit 11,400 159,000 5,900 34,000 390,000 Cash Accounts receivable Prepaid rent Inventory...
-
Hydrocarbons are often represented by line drawings. How is it possible to look at a line drawing and deduce the molecular formula of the compound?
-
What is the source of most hydrocarbons, and what ethical issues are involved in burning hydrocarbons as fuel?
-
Which is a composition reaction and which is not? a. H2 + Cl2 ( 2HCl b. 2HBr + Cl2 ( 2HCl + Br2
-
The waiting times between a subway departure schedule and the arrival of a passenger are uniformly distributed between 0 and 9 minutes. Find the probability that a randomly selected passenger has a...
-
Greenview Dairies produces a line of organic yogurts for sale at supermarkets and specialty markets in the Southeast. Economic conditions and changing tastes have resulted in slowing demand growth....
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Inc. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2019, are as indicated. Described here are...
-
If you were team leader how would you break up this assignment for 4 people to complete? Group Case Analysis Parts 4, 5, and 6 IV. STRATEGY IMPLEMENTATION. (How are you going to do what you want to...
-
A genetic experiment with peas resulted in one sample of offspring that consisted of 440 green peas and 166 yellow peas. Construct a 90% confidence interval to estimate of the percentage of yellow...
-
A 0.15-kg baseball is pitched with a speed of 35 m/s (78 mph). When the ball hits the catcher's glove, the glove moves back by 5.0 cm (2 in.) as it stops the ball. (a) What was the change in momentum...
-
Juarez worked for Westarz Homes at construction sites for five years. Bever was a superintendent at construction sites, supervising subcontractors and moving trash from sites to landfills. He...
-
For a system of atoms (in equilibrium) having two energy levels, show that at high temperatures where k B T >> E j - E i , the number densities of the two states tend to become equal.
-
Radiation at 21 cm pours down on the Earth from outer space. Its origin is great clouds of hydrogen gas. Taking the background temperature of space to be 3.0 K, determine the ratio of the transition...
-
With the Example 13.7 in mind, determine the average power per cubic meter radiated by the Nd:YAG laser rod, given that the transition occurs with an upper-level lifetime of 230 s.
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


