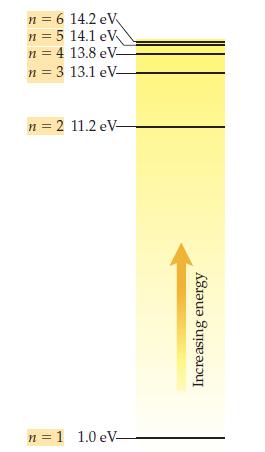
Examine the energy-level diagram for hydrogen shown above, and answer the following questions: (a) If an electron
Question:
Examine the energy-level diagram for hydrogen shown above, and answer the following questions:
(a) If an electron is in the n = 1 shell, what is the electron’s scaled energy in electronvolts?
(b) If an electron is in the n = 2 shell, what is the electron’s scaled energy in electronvolts?
(c) How much energy would it take to transfer an electron from the n = 1 shell to the n = 2 shell?
(d) Would it take the same, more, or less energy than your answer to (c) to take an electron from the n = 2 shell to the n = 3 shell? Why?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





