Fill in the table: Wavelength (m) 7.85 x 10-12 m Wavelength (nm) 2602.0 nm Energy (J) 5.65
Question:
Fill in the table:
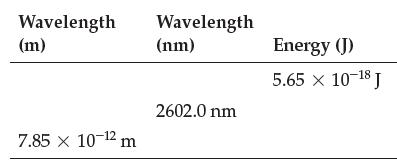
Transcribed Image Text:
Wavelength (m) 7.85 x 10-12 m Wavelength (nm) 2602.0 nm Energy (J) 5.65 × 10-18 J
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The table you sent shows the wavelength and energy of a wave The wavelength is given in two units me...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Any work on how you arrived at the answer would be appreciated! :) Thanks!
-
Discuss two strategies that can be used for leading change. How do these strategies increase stakeholder support and create momentum for a change initiative to be successful? Why might you want to...
-
Fill in the table below for the following zero-coupon bonds, all of which have par values of$1,000. Bond-Equivalent Price Maturity (years Yield to Maturity $400 $500 $500 20 20 10 10 10 10% 8% 8% $400
-
Muscles: Identify the name of the muscle, the origin, the insertion, and the action of the muscle labeled on the models. iliopsoas (psoas major and iliacus) gluteus maximus gluteus medius sartorius...
-
Clark Kent, Inc., buys crypton for $0.80 a gallon. At the end of processing in Dept. 1, crypton splits off into products A, B, and C. Product A is sold at the split-off point with no further...
-
On January 1, 2013, Caswell Company signs a 10-year cancelable (at the option of either party) agreement to lease a storage building from Wake Company. The following information pertains to this...
-
According to Gray, how do societal values affect national accounting systems? LO4
-
Using the information provided here for the Airport Enterprise Fund of the City of Demere, prepare a statement of revenues, expenses, and changes in fund net position for 20X3. Charges for services ....
-
Please answer the question as soon as possible like in 20 minutes Haleiwa Products had the following transactions in March. Prepare journal entries for these transactions assuming Haleiwa uses a...
-
Electromagnetic radiation emitted by magnesium has a wavelength of 285.2 nm. (a) Is this radiation visible to the eye? (b) What is the energy of this radiation?
-
What is the wavelength in nanometers of infrared light for which l = 2.50 10 5 m? How many times longer is this wavelength than red light that has a wavelength of 750 nm?
-
What are the advantages and disadvantages of Best Buys customer centricity strategy?
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
In Problems a-c, give a proof of the indicated property for two-dimensional vectors. Use u = (u1, u2), v = (v1, v2), and w = (w1, w2). a. (a + b)u = au + bu b. u v = v u c. c(u v) = (cu) v
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
Derive the ground-state term symbols for the following configurations: a. d 5 b. f 3 c. p 4
-
As discussed in Chapter 20, in a more exact solution of the Schrödinger equation for the hydrogen atom, the coordinate system is placed at the center of mass of the atom rather than at...
-
What are the levels that arise from a 4 F term? How many states are there in each level?
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...

Study smarter with the SolutionInn App


