For those reactions in Problem 10.58 that are electron-transfer reactions: (a) Indicate which atoms get oxidized and
Question:
For those reactions in Problem 10.58 that are electron-transfer reactions:
(a) Indicate which atoms get oxidized and which atoms get reduced.
(b) Indicate which reactant is the oxidizing agent.
(c) Indicate which reactant is the reducing agent.
Data from Problem 10.58
Which of the following are electron-transfer reactions?
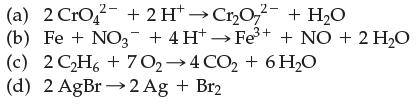
Transcribed Image Text:
(a) 2 CrO2 + 2H+→Cr₂O72- + H₂O (b) Fe + NO3 + 4H+→Fe³+ + NO + 2 H₂O (c) 2C2H6+7O,→4CO,+6H,O (d) 2AgBr→2Ag + Br2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
For those reactions in Problem 10.56 that are redox reactions: (a) Indicate which atoms get oxidized and which atoms get reduced. (b) Indicate which reactant is the oxidizing agent. (c) Indicate...
-
From the Nerst equation E = E0 + RT/nF ln a... show that for a pH electrode that an uncertainty in the measurement of the potential leads to an error of 4% in the measurement of the pH.
-
The following half reactions play important roles in metabolism. 1/2 O2 1 2H+ + 2e H2O NADH + H+ NAD+ + 2H+ + 2e- Which of these two is a half reaction of oxidation? Which one is a half reaction of...
-
The total cost of 2 chair and 1 table is 210 dollars. The total cost of 1 chair and 2 tables is 285 dollars. What is the cost of 1 chair?
-
The controller of Sedona Housewares Inc. instructs you to prepare a monthly cash budget for the next three months. You are presented with the following budget information: The company expects to sell...
-
Courts have tended to be protective of managers who fight against hostile takeovers, even if the bidder is offering a price much higher than the market value of the shares. Why? Do the courts have...
-
How can a global retailer ensure that they adapt to conditions in an overseas market while retaining their unique features and value proposition? LO1
-
Flanagan Company reports the following for the month of June. Instructions(a) Compute the cost of the ending inventory and the cost of goods sold under (1) FIFO and (2) LIFO.(b) Which costing method...
-
Which of the following is an example for public restrictions on land use? Encroachments Easement Zoning CCR
-
The following reaction is responsible for producing electricity in your car battery (often called a lead storage battery): (a) Assign an oxidation state to each atom. (b) Identify the atom that gets...
-
Which of the following are redox reactions? (a) 2Na + 2H 2 O 2NaOH + H 2 (b) MgBr 2 + 2NaF MgF 2 + 2NaBr (c) 2CO + O 2 2CO 2 (d) SO 2 + H 2 O H 2 SO 3
-
Form a study group of four to six persons who are to act out the role of the finance director and related staff on the accounting team of a company planning to open a new supermarket chain at an...
-
Find the unknown angle measures. 49 60 Drawing is not to scale. I = y = In S
-
Q5 For this question, use data from only restaurants with between 50 and 60 items in the data set. Predict total fat from cholesterol, total carbs, vitamin a, and restaurant. Remove any...
-
A meteorologist believes that there is a relationship between the daily mean windspeed, w kn, and the daily mean temperature, t C. A random sample of 9 consecutive days is taken from past records...
-
Suppose k(x) = f(g(h(x))). Given the table of values below, determine k' (1). g(x) h(x) f'(x) g'(x) h'(x) x f(x) 1 -6 -3 3 6 -6 -6 3 -3 4 1 -7 -2 5 4 -2 7 3 1 -7 -8
-
In a research study women with metastatic stomach cancer responded to the Symptom Distress Scale and the Profile of Mood States. A correlation coefficient was reported: r = 0.5, p = 0.03. How would...
-
Let A be a 5 3 matrix of rank 3 and let {x1,x2,x3} be a basis for R3. (a) Show that N(A) = {0}. (b) Show that if y, = Ax1, y2 = Ax2, and y3 = Ax3, then y1, y2, y3 are linearly independent. (c) Do...
-
Catalytic hydrogenation of naphthalene over PdC results in rapid addition of 2 moles of H 2 . Propose a structure for this product.
-
A candle flame is 18.0 cm in front of a thin positive lens. Its image appears three times farther away from the lens than if the same candle were on a very distant mountain. Determine the lenss focal...
-
What must the focal length of a thin negative lens be for it to form a virtual image 50 cm away (measured from the lens) of an ant located 100 cm away (measured from the lens)? Given (just as a...
-
An LED is on the central axis 30.0 cm in front of a thin lens. The resulting image, which is virtual, is 10.0 cm from the lens. Determine the focal length of the lens. Using Table 5.3, explain why...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


