How many valence electrons does each atom in the methane molecule have? Double-count shared electrons. HTCIH
Question:
How many valence electrons does each atom in the methane molecule have? Double-count shared electrons.
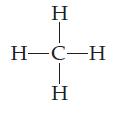
Transcribed Image Text:
HTCIH Η H-C-H Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The carbon atom has eight val...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Explain subsequent event and why these are important? Explain with the help of example
-
How many valence electrons does a carbon atom normally have? How many valence electrons does it have in the methane molecule? How many electrons did it gain, and how many covalent bonds to hydrogen...
-
How many valence electrons does each of the following atoms have? (a) Na (b) Cl (c) Si (d) B (e) Ne (f) N
-
"One need not be concerned with reliability and validity in applied marketing research." Discuss this statement as a small group.
-
Accenture is a firm that provides a wide range of consulting and services to organizations worldwide. With more than 170,000 employees, the firm has clients in 120 countries that receive many HR and...
-
Use the Allstott 2014 income statement that follows and the balance sheet from exercise S13-6 to compute the following: a. Allstott's rate of inventory turnover and days inventory outstanding for...
-
Construct and interpret separate ROI charts for the four models. (Extra credit: Find a way to construct a single ROI chart comparing the four models.) Which model is preferred, and why?
-
Following is information on two alternative investments being considered by Jin Company. The company requires a 10% return from its investments. For each alternative project compute the (a) Net...
-
The next dividend payment by HG Enterprises will be $2.35 per share. The dividends are anticipated to maintain a 2.5 percent growth rate forever. The stock currently sells for $54.60 per share. What...
-
A covalent bond: (a) Is the name given to the attractive force between atoms in a molecule. (b) Is strong because the electrons in the bond can be attracted to two nuclei instead of just one. (c) Is...
-
Predict the formula of the compound that forms between silicon (Si) and bromine (Br).
-
Evaluate the following integral. CT/2 V1 cos 2x dx
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
Plot the graph of the hypocycloid For appropriate values oft in each of the following cases: a. a = 4, b = 1 b. a = 3, b = 1 c. a = 5, = 2 d. a = 7, b = 4 Experiment with other positive integer...
-
How does the organizational structure of an MNC influence its strategy implementation?
-
Consider rotation about the C~C bond in ethane. A crude model for torsion about this bond is the free rotor model where rotation is considered unhindered. In this model the energy levels along the...
-
Inspection of the thermodynamic tables in the back of the text reveals that many molecules have quite similar constant volume heat capacities. a. The value of C V, m for Ar (g) at standard...
-
The molar constant volume heat capacity for I 2 (g) is 28.6 J mol 1 K 1 . What is the vibrational contribution to the heat capacity? You can assume that the contribution from the electronic degrees...
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


