How would you classify the molecule in the left beaker? HC OH + || HC 0
Question:
How would you classify the molecule in the left beaker?
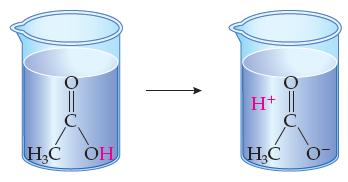
Transcribed Image Text:
HC OH н+ || HC 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
The molecule in the left beaker is water H2O Water is a covalent molecule meaning that the atoms ...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
How would you classify the layout of a large grocery store? Why do you think it is laid out this way? Can you think of any way to improve the layout of a conventional grocery store? Explain your...
-
Haniya and Aleena commenced in partnership on 1st February 2020 and prepared their first accounts for the period ending 31st July 2021 which reported a Tax Adjusted Trading Partnership Profit of...
-
Though the McDonalds (MCD) menu of hamburgers, cheeseburgers, the Big Mac, Quarter Pounder, Filet-O-Fish, and Chicken McNuggets is easily recognized, McDonalds financial statements may not be as...
-
Hunt Manufacturing Company makes tents that it sells directly to camping enthusiasts through a mail-order marketing program. The company pays a quality control expert $72,000 per year to inspect...
-
A person read that the average number of hours an adult sleeps on Friday night to Saturday morning was 7.2 hours. The researcher feels that college students do not sleep 7.2 hours on average. The...
-
What is the conceptual relationship between the discount rates at the marketable minority (Rmm) and nonmarketable minority (Rhp) levels of value?
-
Tess is the development manager for the Isabelle Stewart Gardner Museum in Boston. She was in the middle of a large campaign to raise $50 million for a building expansion project. Her development...
-
(10 points) ORENG STUDENT FACULTY OF BUSINESS AND 3. The following balances are taken from the trial balance of QMX Company as of 31.10.2021 Determine the value of cash. Accounts Fading value (TL)...
-
In the chapter, we saw the combustion reaction for methane. This was actually a complete combustion reaction because carbon ended up as carbon dioxide. If the same reaction is carried out where the...
-
Using molecular formulas, write a balanced equation for the following gas-phase reaction. Then translate the balanced reaction into an English sentence. H" H H + Cl-Cl H H H Cl +H-Cl Cl
-
In the study of spur gears in contact, the equation is used. Solve for r 1 2 kC = R R + r = r - A
-
Problem 2-26 (Static) Complete the balance sheet using cash flow data LO 2-2, 2-3, 2-5, 2-6 Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together...
-
Consider the following potential events that might have occurred to Global Conglomerate on December30, 2018. For eachone, indicate which line items inGlobal's balance sheet would be affected and by...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Jamonit Ltd is a non-group employer which paid wages of $136,000 in the Northern Territory during March 2021. The company does not pay wages in any other state. Calculate the payroll tax payable in...
-
Following is a partially completed balance sheet for Epsico Inc. at December 31, 2019, together with comparative data for the year ended December 31, 2018. From the statement of cash flows for the...
-
What product would be formed from the SN2 reaction of the following? a. (R)-2-bromobutane and hydroxide ion b. (S)-3-chlorohexane and hydroxide ion c. 3-iodopentane and hydroxide ion
-
Ask students to outline the reasons why the various elements of culture (social structures and control systems, language and aesthetics, religion and other belief systems, educational systems, etc.)...
-
Compute the specific weight of air at 80 psig and 75F.
-
Express the pressure of 925 Pa in psi.
-
The static pressure in a gas pipe is measured to be 925 Pa. Express this pressure in in H 2 O.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


