In WorkPatch 14.8, why are the rates of the forward and reverse reactions greater at the new
Question:
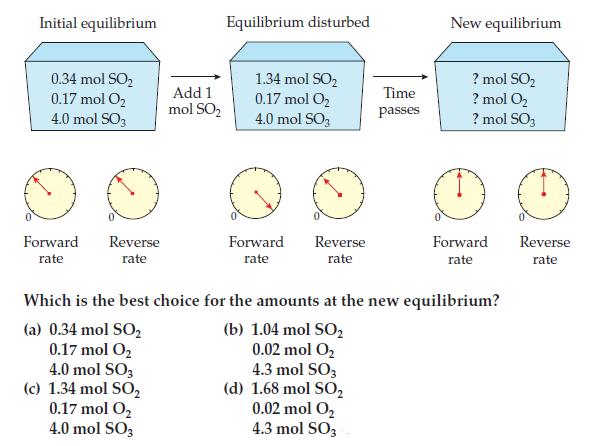
In WorkPatch 14.8, why are the rates of the forward and reverse reactions greater at the new equilibrium than at the initial equilibrium?
Data from WorkPatch 14.8
Transcribed Image Text:
Initial equilibrium 0.34 mol SO₂ 0.17 mol O₂ 4,0 mol SO3 Forward rate Reverse rate Add 1 mol SO₂ 4.0 mol SO3 (c) 1.34 mol SO₂ 0.17 mol O₂ 4.0 mol SO3 Equilibrium disturbed 1.34 mol SO₂ 0.17 mol O₂ 4.0 mol SO3 Forward rate Reverse rate Time passes New equilibrium ?mol SO₂ ? mol O₂ ? mol SO3 Forward rate Which is the best choice for the amounts at the new equilibrium? (a) 0.34 mol SO₂ (b) 1.04 mol SO₂ 0.17 mol O₂ 0.02 mol O₂ 4.3 mol SO3 (d) 1.68 mol SO₂ 0.02 mol O₂ 4.3 mol SO3 Reverse rate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
In both cases the forward rate equals the reverse rate This must be true beca...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Identify each of the following statements as either true or false. (a) At equilibrium the rates of the forward and reverse reactions are equal. (b) When a reaction reaches equilibrium the forward and...
-
Why are the rates of the SN2 reactions of ethyl bromide and methyl bromide given in Table 10.4 so slow?
-
The enzyme Stell participates in several distinct MAP kinase signaling pathways in the budding yeast S. cerevisiae. What is the substrate for Ste 11 in the mating factor signaling pathway? When a...
-
Identifying Transactions as Investing or Financing Activities on the Statement of Cash Flows For the transactions in M2-5, identify each as an investing (I) activity or financing (F) activity on the...
-
How does semiotics help marketers understand consumer behavior?
-
What factors create a foreign exchange gain on a foreign currency transaction? What factors create a foreign exchange loss? LO9
-
Paul Adams owns a health club in downtown Los Angeles. He charges his customers an annual fee of $500 and has an existing customer base of 600. Paul plans to raise the annual fee by 6 percent every...
-
The following payroll data pertain to four employees of the Comfort Shoe Corporation. Compute the gross earnings of each employee. Employee Hours Worked Hourly Rate Overtime Rate Gross Earnings Cindy...
-
Suppose the reaction has come to equilibrium. Which of the following are true? (a) Adding C(s) will shift the reaction to the right. (b) Adding CO(g) will shift the reaction to the right. (c) Adding...
-
Suppose you have a vessel containing an endothermic reaction at equilibrium: When you put the vessel in a freezer, which way does the reaction shift? Why? Heat + Reactants Products Endothermic...
-
Refer to check digit codes in which the check vector is the vector c = [1, 1, . . . , 1] of the appropriate length. In each case, find the check digit d that would be appended to the vector u. u [1,...
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
Show that if Q is any 4 4 orthogonal matrix then ||Q||2 = 1 and ||Q||F = 2.
-
1A. If the researcher is concerned about the number of variables, the nature of the analysis, and completion rates, then, he/she is at which stage of the sampling design process (Figure 11.1 in the...
-
For the hydrometer designed in Problem 5.13, what will be the specific gravity of the fluid in which the hydrometer would float at the top mark? Cross section of hull Water surface X= 1,06 m + cg +...
-
For the hydrometer designed in Problem 5.13, what will be the specific gravity of the fluid in which the hydrometer would float at the bottom mark? Cross section of hull Water surface X= 1,06 m + cg...
-
A buoy is to support a cone-shaped instrument package, as shown in Fig. 5.22. The buoy is made from a uniform material having a specific weight of 8.00 lb/ft 3 . At least 1.50 ft of the buoy must be...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


