Rank the molecules below in order of increasing boiling point. Cl [T HCH H-C-F 1 -F2 F
Question:
Rank the molecules below in order of increasing boiling point.
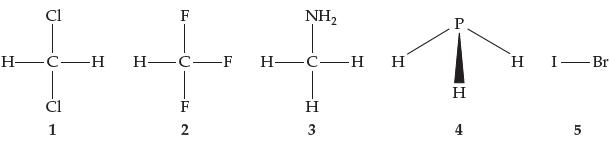
Transcribed Image Text:
Cl [T H—C—H H-C-F 1 -F2 F NH₂ H-CH H H 3 P H 4 H I-Br 5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The molecules in the image can be ranked in order of increasing boiling point as follows 1 ClFNH2 2 ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Rank the following molecules in order of increasing boiling point (without looking up the real values!): (a) 3-methylheptane; (b) Octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.
-
(a) Rank these molecules in order of increasing boiling point: (b) State your reason for the order you chose in part (a). H H-C-OH H H-H H H-C-H H H-Cl
-
(a) Rank these molecules in order of increasing boiling point: (b) State your reason for the order you chose in part (a). Br Br-C-Br Br Br Br H H-C-H H CI-CI
-
March 31, 2014, adjusted trial balance for Brenner Climbing Adventures has been alphabetized as follows: Required Journalize the closing entries. No. Account Debit Credit $ 2,600 168 Accumulated...
-
You have recently assumed the role of HR Manager in your company. In reviewing the company records, you note that the job descriptions were last updated 5 years ago. The Company President has taken...
-
Janet is considering transferring assets valued at $9 million to an irrevocable trust (yet to be created) for the benefit of her son, Gordon, age 15, with Farmers Bank as trustee. Her attorney has...
-
Generate numerical summaries for the clusters. For example, generate a cluster mean summary.
-
Cost-Cutting Proposals Chatman Machine Shop is considering a four-year project to improve its production efficiency. Buying a new machine press for $530,000 is estimated to result in $205,000 in...
-
Allen is an employee of the foreign subsidiary of a United States base company who lives and work in the United Kingdom. according to FATCA, how much of his wages may Allen exclude from United...
-
Some bacteria have evolved in such a way to render the antibiotic Vancomycin ineffective. How did they accomplish this (make sure to explain the role of intermolecular forces)?
-
For each part of this problem, redraw both of the molecules, orienting them in a way that allows you to clearly show (using a dotted line) a hydrogen bond between them. If it is not possibleto form a...
-
In the 1980s, the financial press raised the public consciousness by publicizing a series of audit failures and financial failures, most notably in the banking and government securities industries....
-
Using a ruler and set squares only, construct the following shapes: a. b. c. d. 5cm 5cm
-
The marketing department has just forecast that 10,000 units of item 778 will be ordered in the next fiscal year. Based on the marketing department's forecast and noting that the seasonal relative...
-
Following are interaction plots for three data sets. Which data set has the largest interactions? Which has the smallest? A B C
-
From your local chamber of commerce, obtain the population figures for your city for the years \(1980,1990,2000\), and 2010. Find the rate of growth for each period. Forecast the population of your...
-
A mass \(m\) is attached at the midpoint of a stretched wire of area of cross-section \(A\), length \(l\), and Young's modulus \(E\) as shown in Fig. 13.29. If the initial tension in the wire is...
-
The path of a projectile fired from level ground with a speed of v0 feet per second at an angle a with the ground is given by the parametric equations x = (v0 cos )t, y = - 16t2 + (v0 sin )t a. Show...
-
Air pollution generated by a steel mill is an example of a) a positive production externality. b) a negative production externality. c) a public good. d) the free-rider problem. State and local taxes...
-
Determine the mean free path at 500. K and 1 atm for the following: a. Ne b. Kr c. CH 4 Rather than simply calculating the mean free path for each species separately, instead develop an expression...
-
Consider the diagram of a molecular beam apparatus provided in the text. In the design of the apparatus, it is important to ensure that the molecular beam effusing from the oven does not collide with...
-
A comparison of ave , mp , and rms for the Maxwell speed distribution reveals that these three quantities are not equal. Is the same true for the one-dimensional velocity distributions?
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


