Redraw each structure to get the electron groups as far apart as possible. What angles did you
Question:
Redraw each structure to get the electron groups as far apart as possible. What angles did you use?
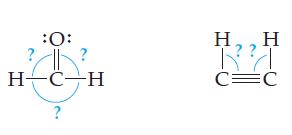
Transcribed Image Text:
:O: ? H CH ? H RA C=C H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
120 120 H ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Fill in the missing mass number and atomic number for each of these particles or types of radiation. alpha (?) He beta (?) e gamma y neutron n
-
Do you agree that the company is guilty of illegal actions? Why or why not?
-
Universal Gizmo (UG) operates a large number of plants that produce gizmos using a special technology. Each plant produces exactly 100 gizmos per day using 5 gizmo presses and 15 workers. Explain why...
-
16-3. La toma de decisiones y compras centralizadas constituyen una ventaja de la forma de propiedad llamada _______________.
-
Hoffman Containers manufactures a variety of boxes used for packaging. Sales of its Model A20 box have increased significantly to a total of 480,000 A20 boxes. Hoffman has enough existing production...
-
Microsoft is reinventing themselves in 2015. The first questions they addressed in a recent board meeting is, "how should we define Microsoft in 2015 and which businesses should we target for faster...
-
Describe the geometry of the electron groups and name the molecular shape resulting from that geometry. Also, draw the molecule, and label the size of all bond angles in your drawing. CO 2 .
-
The diagram below shows how bent water molecules in solid ice orient themselves with respect to each other. Notice the large openings in the lattice of water molecules. (a) Discuss why it takes...
-
When a baseball thrown at 85 miles per hour is hit by a bat swung at x miles per hour, the ball travels 6x - 40 feet (This formula assumes that 50 x 90 and that the bat is 35 inches long, weighs 32...
-
Manufacturing company produces $3800 worth of products weekly. If the cost of raw materials to make this product is $400, and the labour cost is $360, calculate the productivity.
-
1-You are a very well-recognized professional in your area, with many years of experience solving international conflicts. There is a company in the middle of two European countries that are fighting...
-
Find the solution u = u(x,y) of the following problem on the set R. u du - 4, (1.4) Ju(0,y) =3y, u(x, 0) = 0. (1.5) ay
-
Scenario A Sports Club 10 Highfield Sports Club has organised a fundraising event. 300 tickets have been sold at a price of $2.50 each. Money taken at the event Percentage of money (E) taken (96)...
-
Shamrock Investments has three divisions (Green, Clover, Seamrog) organized for performance evaluation purposes as investment centers. Each division's required rate of return for purposes of...
-
Find the slope of the tangent line to each of the following curves at 0 = rr/3. a. r = 2 cos b. r = 1 + sin c. r = sin 2 d. r = 4 - 3 cos
-
You continue to work in the corporate office for a nationwide convenience store franchise that operates nearly 10,000 stores. The per- store daily customer count (i.e., the mean number of customers...
-
How many rotational degrees of freedom are there for linear and nonlinear molecules?
-
Assuming 19 F 2 and 35 Cl 2 have the same bond length, which molecule do you expect to have the largest rotational constant?
-
Consider the rotational partition function for a polyatomic molecule. Can you describe the origin of each term in the partition function, and why the partition function involves a product of terms?
-
( US$ millions ) 1 2 / 3 1 / 2 0 1 4 1 2 / 3 1 / 2 0 1 3 1 2 / 3 1 / 2 0 1 2 1 2 / 3 1 / 2 0 1 1 Net income $ 1 4 , 4 3 1 $ 1 2 , 8 5 5 $ 1 0 , 7 7 3 $ 9 , 7 7 2 Depreciation 3 , 5 4 4 2 , 7 0 9 1 ,...
-
net present value of zero
-
Suppose at Time 0 a dealer buys $100 par of a 4%-coupon 30-year bond for a price of par and posts the bond as collateral in a repo with zero haircut. The repo rate is 5%. Then, 183 days later, the...

Study smarter with the SolutionInn App


