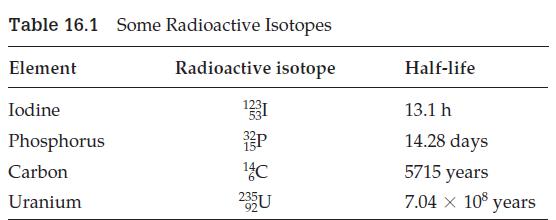
The radioactive isotope of iodine 123 53 I is used to treat thyroid disease. Suppose a patient
Question:
The radioactive isotope of iodine 12353I is used to treat thyroid disease. Suppose a patient is given a 30 mg dose. How much will be left in the patient after 39.3 h? (See Table 16.1.)
Transcribed Image Text:
Table 16.1 Some Radioactive Isotopes Radioactive isotope Element Iodine Phosphorus Carbon Uranium 1331 P ¹4 C 235U Half-life 13.1 h 14.28 days 5715 years 7.04 × 108 years
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
Because 393 h i...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A breeder reactor converts stable uranium-238 into the isotope plutonium-239. The decay of this isotope is given by A(t) = A0e-0.00002876t where A(t) is the amount of the isotope at time t, in years,...
-
The radioactive isotope of iodine, 1- 1 35, decays into the radioactive isotope Xe- 1 35 of xenon; this in tum decays into another (stable) product. The half-lives of iodine and xenon are 6.7 hours...
-
Suppose a radioactive material has a half-life of 100 years. What is the decay rate ? Starting with an initial sample of 100 grams, how much will be left after 10 years? 100 years? 1,000 years?
-
Will restricting imports affect exports and export-related jobs? How?
-
A dialysis clinic provides two types of treatment for its patients. Hemodialysis (HD), an in house treatment, requires that patients visit the clinic three times each week for dialysis treatments....
-
Organophosphate (OP) compounds are used as pesticides. However, it is important to study their effect on species that are exposed to them. In the laboratory study Some Effects of Organophosphate...
-
Let = 1 be the correlation process of two Brownian motions B1 and B2. Set B 1 = B1. Define B 2 by B 20 = 0 and dB 2 = 1 1 2 (dB2 dB1). Show that B 1 and B 2 are independent Brownian motions. Note:...
-
On the first day of the fiscal year, a company issues a $1,500,000, 9%, five-year bond that pays semiannual interest of $67,500 ($1, 500,000 9% ), receiving cash of $1,334,398. Journalize the bond...
-
The Pharos Fund is valued at 750,000 at the start of the month. On day 22, a contribution of 20,000 is made. At the end of the month, the Fund is worth 1,266,513. Compute the money weighted rate of...
-
The nuclear process which constantly produces 14 C in the Earths atmosphere is illustrated below, along with the mechanism of its incorporation into the food chain. What is the major assumption upon...
-
(a) What does the plot say about the stability of the nucleus of the iron isotope 56 26 Fe? (b) What is the total binding energy for the 56 26 Fe nucleus in kilojoules per mole of atoms?
-
Graph y = f(x), y = f -1 (x), and y = x in a square viewing rectangle such as [-4.7, 4.7, 1] by [-3.1, 3.1, 1]. I-XA = (x)f
-
Gary Tuttle has Citiwide Insurance with 100% coverage after a $25.00 copay on office visits. His services today include an office visit ($62.00), urinalysis with differential ($65.00) and a Treadmill...
-
The Elgin Golf Dutton Golf Merger Elgin Golf Inc. has been in merger talks with Dutton Golf Company for the past six months. After several rounds of negotiations, the offer under discussion is a...
-
f ( x ) = x ^ 3 - 3 x ^ 2 - 2 4 x + 5 6 find all critical numbers
-
Suppose a beam of electrons is aimed at two slits in a slide placed in front of a screen. After a short time, the screen looks like the one at the right. a. What evidence does the picture give that...
-
On January 1, Mitzu Company pays a lump-sum amount of $2,700,000 for land, Building 1, Building 2, and Land Improvements 1. Building 1 has no value and will be demolished. Building 2 will be an...
-
Give two sets of alkyl bromide and alkene that could be used in a Heck reaction to prepare the following compound: CH3C CH= CH OCH3
-
In your audit of Garza Company, you find that a physical inventory on December 31, 2012, showed merchandise with a cost of $441,000 was on hand at that date. You also discover the following items...
-
The switch in Fig. 16.49 moves from position A to position B at t = 0 (please note that the switch must connect to point B before it breaks the connection at A, a make before break switch). Determine...
-
The switch in the circuit of Fig. 16.47 has been closed for a long time but is opened at t = 0. Determine i(t) for t > 0. i(t) 2 40 V t = 0 -/4 1/2
-
Find the voltage across the capacitor as a function of time for t > 0 for the circuit in Fig. 16.45 . Assume steady-state conditions exist at t = 0 . 5 t = 0 1 0.25 60 V (+ +1
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


