Two different compounds, both consisting of sodium (Na) and oxygen (O), were analyzed. The data are given
Question:
Two different compounds, both consisting of sodium (Na) and oxygen (O), were analyzed. The data are given below:
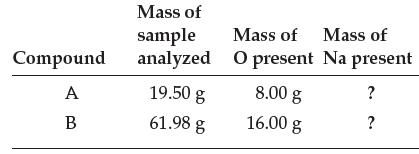
(a) Fill in the last column of the table.
(b) Calculate the %Na and %O for both compounds.
Transcribed Image Text:
Compound A B Mass of sample analyzed 19.50 g 61.98 g Mass of Mass of O present Na present ? ? 8.00 g 16.00 g
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To fill in the last column of the table we need to calculate the mass of sodium Na prese...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The following questions concern Poisson regression models fit to fictitious follow-up study data in which rates of disease are modeled as a function of age and smoking status. The SAS program codes...
-
Which of the following is an example of how managers use production cost reports to control costs? A. providing cost of goods sold for the income statement B. determining if newer, more efficient...
-
In 1950, J. R. Clarkson founded a family-owned industrial valve design and manufacturing company in Sparks, Nevada. For almost a half century, the company, known as the Clarkson Company, worked on...
-
1. What options does Personal Trainer have for developing a new system? What are some specific issues and options that Susan should consider in making a decision? 2. Susan has been asked to prepare a...
-
The following information was summarized from the balance sheets of the Coca-Cola Company and Subsidiaries at December 31, 2008, and PepsiCo Inc. and Subsidiaries at December 27, 2008: Required 1....
-
Redo Exercise 5.2.1 using (i) The weighted inner product (v, w) = 3 v1 w1 + 2 v2 w2 + v3 w3: (ii) The inner product induced by the positive definite matrix 012
-
Explain contractual entry strategies. LO.1
-
What if Johns neighbor made his promise to help reassemble the garage at the time he and John were moving it to Johns property, saying, Since you helped me take it down, I will help you put it back...
-
1.When interest rates are low, some automobile dealers offer loans at 0% APR, as indicated in a 2016 advertisement by a prominent car dealership, offering zero percent financing or cash back deals on...
-
Bromine (Br) has two abundant isotopes, one with 44 neutrons and the other with 46 neutrons. Give the full atomic symbols for both isotopes.
-
An electromagnet bends the path of the fast moving ions in a mass spectrometer so they can reach the detector. The strength of an electromagnets magnetic field depends on the how much voltage is...
-
Define liquidity, financial flexibility, and operating capability.
-
Coronado Corporation accumulates the following data relative to jobs started and finished during the month of June 2025. Costs and Production Data Actual Standard Raw materials unit cost $2.20 $2.10...
-
Consider the following labor statistics for the adult population (age 16 and older) in Norway displayed in the table below (all numbers in millions). Employed 110 13 Not in Labor Force 75 Unemployed...
-
Blossom Variety Store uses the LIFO retail inventory method. Information relating to the computation of the inventory at December 31, 2026, follows: Cost Retail Inventory, January 1, 2026 $147,000...
-
Use polynomial division to show that the general expression for the factors of the difference of two cubes, x 3 - y 3 = ( x - y ) ( x 2 + xy + y 2 ) , is correct
-
A beam has a lenght of L = 9 m long and carrying the uniformly distributed load of w = 3 kN/m. w kN/m A RA a) Calculate the reaction at A. RA= KN L RB b) Calculate the maximum bending moment. Mmax=...
-
Suppose that components have weights that are independent and uniformally distributed between 890 and 892. (a) Suppose that components are weighed one by one. What is the probability that the sixth...
-
Design a circuit which negative the content of any register and store it in the same register.
-
Identify whether each of the following compounds exhibits a molecular dipole moment. For compounds that do, indicate the direction of the net molecular dipole moment: a. CHCl 3 b. CH 3 OCH 3 c. NH 3...
-
Which of the following compounds has the larger dipole moment? Explain your choice: CHCl 3 or CBrCl 3
-
The specific rotation of ephedrine in ethanol (at 20C) is -6.3. A chemist prepared a mixture of ephedrine and its enantiomer, and this mixture had a specific rotation of -6.0. Calculate the % ee of...
-
A company sells two products. Assuming the same sales mix as shown below, how many units of Product A must be sold to breakeven? Product A Product B Total Units 100,000 150,000 250,000 Sales $300,000...
-
South Sea Baubles has the following (incomplete) balance sheet and income statement. BALANCE SHEET AT END OF YEAR (Figures in $ millions) Assets 2015 2016 Liabilities and Shareholders' Equity 2015...
-
When the investor pays $100,000 to acquire 40% of a company's outstanding voting shares at a time when the fair value of the company's net assets are $175,000, the resulting goodwill amount is...

Study smarter with the SolutionInn App


