Using your knowledge of VSEPR theory and its preferred bond angles, suggest a reason for the fact
Question:
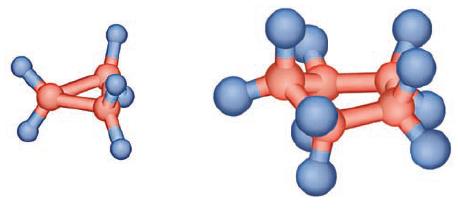
Using your knowledge of VSEPR theory and its preferred bond angles, suggest a reason for the fact that the cyclopropane molecule (pictured below) is not a very stable molecule and is highly reactive, while the cyclopentane molecule is not nearly as reactive or unstable.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The cyclopropane molecule is not very stable and is highly reactive because of its ring strainRing strain is a type of energy that is caused by the de...View the full answer

Answered By

Keziah Thiga
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagirism), well-researched and critically analyzed papers.
4.90+
1504+ Reviews
2898+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The explosion of an atomic bomb releases many radioactive isotopes, including strontium-90. Considering the location of strontium in the periodic table, suggest a reason for the fact that this...
-
The kinetic data for the radical chain chlorination of several cycloalkanes (see the adjoining table) illustrate that the C-H bonds of cyclopropane and, to a lesser extent, cyclobutane are somewhat...
-
The governance of an enterprise is the sum of those activities that make up the internal regulation of the business in compliance with the obligations placed on the firm by legislation, ownership and...
-
Not sure if this note is applicable to the problem? (A2) Prove there is a bijection between any two countably infinite sets.
-
South-Central Publishing Company prepares income statements segmented by divisions, but the chief operating officer is not certain about how the company is actually performing. Financial data for the...
-
Refer to P1-5A and add the following requirement. Prepare a letter to the president of the company, Shelly Phillips, describing the changes you made. Explain clearly why net income is different after...
-
Determine current transportation needs related to new and used motor vehicles and compare buying and leasing options. Your Long-Term Financial Planning Activities
-
Granite Stone Creamery sold ice cream equipment for $ 16,000. Granite Stone originally purchased the equipment for $ 90,000, and depreciation through the date of sale totaled $ 71,000. What was the...
-
Harp Brewing received a bankruptcy notice from their customer Jerry. Musing an allowance method, the entry to write-off his balance of $1,300 would be: Bad Debts Expense IST 300 Allowance for...
-
What is it about the trigonal bipyramidal shape that distinguishes it from all the other molecular shapes that we have covered?
-
Cubane, C 8 H 8 , is an interesting molecule shown below. Comment on it relative to VSEPR. Then imagine you had dichloro cubane, C 8 H 6 C 12 . Draw all the unique molecules with this formula (you...
-
A pump is capable of developing 4.00 kW of power. How many litres of water per minute can be lifted a distance of 35.0 m? (1 L of water has a mass of 1 kg.)
-
a) Describe the following concepts in the context of organizational development. b) Discuss how these concepts interrelate and support each other within an organizational framework
-
Q2. a) Analyze the importance of communication in the change management process. b) Suggests strategies that a Disaster Management Organization can employ to ensure effective communication during...
-
Q3. a) Explain the following Change Management Models
-
Q3. b) Discuss how each model can be applied in real-world organizational change scenarios.
-
In this question, you will work step-by-step through an optimization problem. A craftsman wants to make a cylindrical jewelry box that has volume, V, equal to 55 cubic inches. He will make the base...
-
Sketch the level curves of ((x, y) = (x + y2) for k = 0, 1, 2, 4.
-
Uniform electric field in Figure a uniform electric field is directed out of the page within a circular region of radius R = 3.00 cm. The magnitude of the electric field is given by E = (4.50 x 10-3...
-
Determine in each of the following cases if the function in the first column is an eigenfunction of the operator in the second column. If so, what is the eigenvalue? a. b. c. sine cos 3x (1/x) d/dx
-
If two operators act on a wave function as indicated by AÌBÌ f(x), it is important to carry out the operations in succession, with the first operation being that nearest to the...
-
Find the result of operating with d 2 /dx 2 + d 2 /dy 2 + d 2 /dz 2 on the function x 2 + y 2 + z 2 . Is this function an eigenfunction of the operator?
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


