Which of the following electron transitions in hydrogen would absorb the largest amount of energy? A. B.
Question:
Which of the following electron transitions in hydrogen would absorb the largest amount of energy?
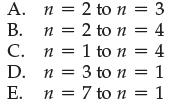
Transcribed Image Text:
A. B. C. D. E. n = 2 to n = 3 n 2 to n = 4 n = 1 to n = 4 n = 3 to n = 1 = n n = 7 to n = 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The electron transition in hydrogen that would absorb the largest amount of energy is E n 1 to n 4 T...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
1. Which of the following electron transitions between two energy states (n) in the hydrogen atom corresponds to the emission of a photon with the longest wavelength? (a) 2 5. (b) 5 2. (c) 5 8....
-
Without doing detailed calculations, indicate which of the following electron transitions requires the greatest amount of energy to be absorbed by a hydrogen atom: from (a) n = 1 to n = 2; (b) n = 2...
-
Without doing detailed calculations, indicate which of the following electron transitions in the hydrogen atom results in the emission of light of the longest wavelength. (a) n = 4 to n = 3; (b) n =...
-
Imagine you are a member of your local school board. You wantan answer to the very simple question, Are students learning?What can you recommend to school principals to answer thequestion?
-
Metropolis Manufacturing Company manufactures a small electric motor that is a replacement part for the more popular gas furnaces. The standard cost card shows the product requirements as follows:...
-
Consider the following relation: CAR_SALE(Car#, Date_sold, Salesperson#, Commission%, Discount_amt) Assume that a car may be sold by multiple salespeople, and hence {Car#, Salesperson#} is the...
-
How does a company determine the fair value of a foreign currency forward contract? How does it determine the fair value of an option? LO9
-
Warmack Machine Shop is considering a four- year project to improve its production efficiency. Buying a new machine press for $410,000 is estimated to result in $155,000 in annual pretax cost...
-
income tax 2 Little Co. is a Canadian controlled private corporation and Large Co. is a public Canadian corporation. Both corporations have a paid-up capital balance of $25,000. Which of these...
-
Consider a 1s 2s electron transition. For which atom would this require shorter wavelength light, H or He? Justify your answer.
-
Predict the formula for the compound aluminum nitride made from the elements aluminum and nitrogen, and explain how you made your prediction.
-
A bottle of pure n-heptane is accidentally poured into a drum of pure toluene in a laboratory. One of the laboratory assistants suggests that since heptane boils at a lower temperature than toluene,...
-
Shire Company's predetermined overhead rate is based on direct labor cost. Management estimates the company will incur $649,000 of overhead costs and $590,000 of direct labor cost for the period....
-
You plan to live 25 years after you retire. You want to withdraw $100,000 each year for 25 years. Your first withdrawal will take place the day after you retire. What is the four annuity formulas...
-
Harwood Company's quality cost report is to be based on the following data: 2021 2022 Depreciation of test equipment $94,000 $95,000 Audits of the effectiveness of the quality system $54,000 $51,000...
-
Cash contribution of 4,000 to the Accounting Society (a charity) Purchase of art object at an Accounting Society Charitable event for $1,200 (FMV $800) Donation of 3-year-old clothing (basis 800; FMV...
-
The government is issuing $100 million in 10 year debt and receives the following bids. $25 million is reserved for non-competitive tenders. At what yield will the non-competitive tenders be issued...
-
Find the area of the triangle with (3, 2, 1), (2, 4, 6), and (-1, 2, 5) as vertices.
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
The muzzle velocity of a rifle bullet is 890.m s 1 along the direction of motion. If the bullet weighs 35 g, and the uncertainty in its momentum is 0.20%, how accurately can the position of the...
-
Why is the probability of finding the harmonic oscillator at its maximum extension or compression larger than that for finding it at its rest position?
-
Why does the energy of a rotating molecule depend on l , but not on m l ?
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


