Write the equilibrium constant expression for (a) 2 FeCl3(s) + 3 HO(g) (b) FeO3(s) + 3 CO(g)
Question:
Write the equilibrium constant expression for
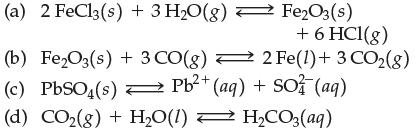
Transcribed Image Text:
(a) 2 FeCl3(s) + 3 H₂O(g) (b) Fe₂O3(s) + 3 CO(g) → (c) PbSO(s) Pb²+ (aq) + SO3(aq) Fe₂O3(s) + 6 HCI(g) 2 Fe(1) + 3 CO₂(g) (d) CO₂(g) + H₂O(1) ⇒ H₂CO3(aq) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a For the reaction 2FeCl3 s 3H2Og Fe2O3s 6HClg the equilibrium constant expression K is ...View the full answer

Answered By

Qurat Ul Ain
Successful writing is about matching great style with top content. As an experienced freelance writer specialising in article writing and ghostwriting, I can provide you with that perfect combination, adapted to suit your needs.
I have written articles on subjects including history, management, and finance. Much of my work is ghost-writing, so I am used to adapting to someone else's preferred style and tone. I have post-graduate qualifications in history, teaching, and social science, as well as a management diploma, and so am well equipped to research and write in these areas.
4.80+
265+ Reviews
421+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider the gas-state reaction (a) Write the equilibrium constant expression for the reaction. (b) Write the reaction in reverse. (c) Write the equilibrium constant expression for the reverse...
-
(a) Write the equilibrium constant expression for the reaction (b) How would the equilibrium be affected if PbI 2 (s) were added? (c) How would the equilibrium be affected if Pb(NO 3 ) 2 (s) were...
-
Compare your equilibrium constant expressions from Problem 14.48(a) and Problem 14.49. (a) Explain how the value of K eq changes for a reaction when you double the reaction (when you multiply it...
-
Tatum is a consultant at R.G. & Company (R.G.), a global consulting firm. She has enjoyed the past few years working at the company. As an ambitious person, she has been focusing on her long-term...
-
Finding Financial Information Refer to the financial statements of American Eagle Outfitters in Appendix B at the end of the book. Required: 1. State the amount of the largest expense on the income...
-
Name 5 reasons people put themselves at risk.
-
An increase in the number of years __________ the holding period return.
-
Multiple Choice Questions 1. Mittelstaedt Inc., buys 60 percent of the outstanding stock of Sherry, Inc. Sherry owns a piece of land that cost $212,000 but had a fair value of $549,000 at the...
-
An investor is considering an investment in a copper mine. The following information is available: The investment will generate an annual pre-tax profit of $50,000 over 3 years. Costs for...
-
As noted in the chapter, the value of K eq for the reaction N 2 (g) + O 2 (g) 2 NO(g) is 0.0017 at 2027 C and 2.3 10 9 at 25 C. (a) Judging from the values of K eq , does this reaction shift to the...
-
What does a catalyst do to the time it takes for a reaction to reach equilibrium? Explain how it does this.
-
How many bytes in State are affected by ShiftRows?
-
15.5 please help will give like if answers r correct Exercise 15-8 (Static) Sales-type lease with selling profit; lessor; calculate lease payments [LO15-3] Manufacturers Southern leased high-tech...
-
When my son was young, he had 8 different plastic dinosaurs to arrange. How many ways could he arrange his 8 dinos? He had favorite dinos, so placing them in proper order was very important. How many...
-
Process P1 init (mutEx); num = 0; loop1 = 0; while (loop1 < 3) wait (mutEx); num num + 1; signal (mutEX); loop1 loop1 + 1; Process P2 loop2 = 0; while (loop2 < 2) wait (mutEx); num num + 10;...
-
PROBLEM 3-5B Following is the chart of accounts of Smith Financial Services: Assets 111 Cash 113 Accounts Receivable 115 Supplies 117 Prepaid Insurance 124 Office Furniture Liabilities 221 Accounts...
-
4. Identify a service you could refer Casey to and write a referral for her (up to 300 words).
-
Beta Products is planning to add another warehouse. Ten products from the entire line are to be stored in the new warehouse. These products will be the A and B items. All C items are to be served out...
-
Ann hires a nanny to watch her two children while she works at a local hospital. She pays the 19 year-old nanny $125 per week for 48 weeks during the current year. a. What is the employers portion of...
-
If the length of the tank in Fig. 4.24 is 1.2 m, calculate the total force on the bottom of the tank. 3 m Tank is Air 200 kPa (gage) 1.2 m long Oil 1.5 m (sg =0.80) Water 2.6 m 2.0 m
-
An observation port in a small submarine is located in a horizontal surface of the sub. The shape of the port is shown in Fig. 4.25. Compute the total force acting on the port when the pressure...
-
A rectangular gate is installed in a vertical wall of a reservoir, as shown in Fig. 4.26. Compute the magnitude of the resultant force on the gate and the location of the center of pressure. Also...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


