Write the equilibrium constant expression for (a) SnO(s) + 2 H(g) Sn(s) + 2 HO(1) (b) H3PO4(aq)
Question:
Write the equilibrium constant expression for
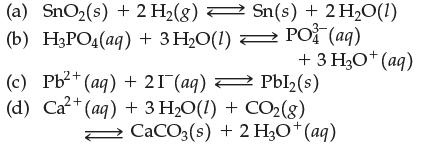
Transcribed Image Text:
(a) SnO₂(s) + 2 H₂(g) Sn(s) + 2 H₂O(1) (b) H3PO4(aq) + 3 H₂O(1) ⇒ PO (aq) 2+ (c) Pb²+ (aq) + 21 (aq) +3HgO*(aq) Pbl₂(s) (d) Ca²+ (aq) + 3 H₂O(1) + CO2(g) CaCO3(s) + 2 H3O+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The equilibrium constant expression for the reaction in the image is Kp SnO2s H2g2 Sns H2Og2 Where K...View the full answer

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider the gas-state reaction (a) Write the equilibrium constant expression for the reaction. (b) Write the reaction in reverse. (c) Write the equilibrium constant expression for the reverse...
-
(a) Write the equilibrium constant expression for the reaction (b) How would the equilibrium be affected if PbI 2 (s) were added? (c) How would the equilibrium be affected if Pb(NO 3 ) 2 (s) were...
-
Compare your equilibrium constant expressions from Problem 14.48(a) and Problem 14.49. (a) Explain how the value of K eq changes for a reaction when you double the reaction (when you multiply it...
-
Consider the following red-black tree. We perform the following sequence of insertions on this tree: 26, 37, 41, 23. Draw the tree after each operation of this sequence (Note that the operations are...
-
Comparing Companies within an Industry Refer to the financial statements of American Eagle Outfitters in Appendix B, Urban Outfitters in Appendix C, and the Industry Ratio Report in Appendix D at the...
-
Define emergency preparedness practices.
-
If a stocks daily price rises from $45 to $50 and retreats to $45, that __________ the daily return calculated using a simple average.
-
The Quick Manufacturing Company, a large profitable corporation, is considering the replacement of a production machine tool. A new machine would cost $3700, have a 4-year useful and depreciable...
-
over time is walmart or chevron company's overall financial performance improving, declining, or is something strange going on ?
-
What is a heterogeneous chemical reaction? Where does a heterogeneous reaction occur?
-
As noted in the chapter, the value of K eq for the reaction N 2 (g) + O 2 (g) 2 NO(g) is 0.0017 at 2027 C and 2.3 10 9 at 25 C. (a) Judging from the values of K eq , does this reaction shift to the...
-
Compute the output slew rate dV od / dt for the op amp in the example in Section 12.6.1. Use the bias currents from the example and C = 1.39 pF.
-
Based on the reading,How to make sure your next product or service launch drives growth (click the underlined link),what stands out to you as the most important factor in a differentiated launch...
-
OM in the News has previously looked at the Waffle House Index, used to measure the damage from hurricanes. The index made the news again for Hurricane Ian. According to the Boston Globe, 40 Waffle...
-
Mixture of persuasive and negative formal I am Elizabeth grinderFirst part email Next part setting up the meeting Final part memo The reader is Robert * do not come off accusatory*** Project TWO:...
-
Anyone who has sampled today's social media offerings has probably experienced this situation: You find a few fascinating blogs, a few interesting people to follow on Twitter, a couple of podcast...
-
a. Begin with a converging lens of focal length f. Place an illuminated object a distance p, in front of the lens. For all positive values of p;: 1. calculate and sketch a graph of the location of...
-
The costs associated with producing, distributing, and selling a domestically produced automotive component to Honda in Japan can be summarized as follows: Transportation costs vary as follows. If...
-
Smiths Family Fashions implemented a balanced scorecard performance measurement system several years ago. Smiths is a locally owned clothing retailer with fashions for men, women, teens, and...
-
A gas-powered cannon shoots projectiles by introducing nitrogen gas at 20.5 MPa into a cylinder having an inside diameter of 50 mm. Compute the force exerted on the projectile.
-
The egress hatch of a manned spacecraft is designed so that the internal pressure in the cabin applies a force to help maintain the seal. If the internal pressure is 34.4 kPa(abs) and the external...
-
A tank containing liquid ammonia at 77F has a flat horizontal bottom. A rectangular door, 24 in by 18 in, is installed in the bottom to provide access for cleaning. Compute the force on the door if...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


