Write the molecular formulas for all potential precipitates in the beaker below. Check your formulas against those
Question:
Write the molecular formulas for all potential precipitates in the beaker below.
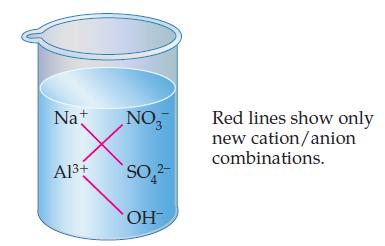
Check your formulas against those at the end of the chapter. Of the three precipitates possible, only Al(OH)3 is insoluble in water. Therefore, the net ionic equation for the precipitation reaction is:
![]()
If you feel comfortable with this three-solution problem, you are ready to tackle any other precipitation problem.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





