A sample of sodium carbonate is treated with 50.0 mL of 0.345 M HCl. The excess hydrochloric
Question:
A sample of sodium carbonate is treated with 50.0 mL of 0.345 M HCl. The excess hydrochloric acid is titrated with 15.9 mL of 0.155 M NaOH. Calculate the mass of the sodium carbonate sample.
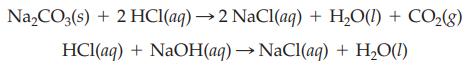
Transcribed Image Text:
Na₂CO3(s) + 2 HCl(aq) → 2 NaCl(aq) + H₂O(l) + CO₂(g) HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
078...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
An antacid tablet contains sodium hydrogen carbonate, NaHCO3, and inert ingredients. A 0.500-g sample of powdered tablet was mixed with 50.0 mL of 0.190 M HCl (hydrochloric acid). The mixture was...
-
An antacid tablet contains sodium hydrogen carbonate, NaHCO 3 , and inert ingredients. A 0.465-g sample of powdered tablet was mixed with 53.3 mL of 0.190 M HCl (hydrochloric acid). The mixture was...
-
A 6.53-g sample of a mixture of magnesium carbonate and calcium carbonate is treated with excess hydrochloric acid. The resulting reaction produces 1.72 L of carbon dioxide gas 28oC at and 743 torr...
-
You bought a share of 3.4 percent preferred stock for $96.82 last year. The market price for your stock is now $98.34. What is your total return for last year?
-
The trial balance of Dealer's Choice Wholesale Company contained the accounts shown at December 31, the end of the company's fiscal year. Adjustment data:1. Depreciation is $8,000 on buildings and...
-
What is the difference between pass-by-value and pass-by-reference?
-
Using the product life cycle, place the products discussed in this case into one of the four stages. Evaluate Mattels strategy for each of these products based on its current life cycle stage. Using...
-
A plate of glass 9.00cm long is placed in contact with a second plate and is held at a small angle with it by a metal strip 0.0800 mm thick placed under one end. The space between the plates is...
-
Miss Krupt, accountant of Dewey, Cheatum and Howe, increased the company's long-term assets to their fair market values on the balance sheet. Which of the following assumptions/principles did she...
-
What is the molar chloride ion concentration resulting from mixing of 50.0 mL of 0.100 M sodium chloride and 25.0 mL of 0.100 M potassium chloride?
-
What is the molar sodium ion concentration resulting from mixing of 50.0 mL of 0.100 M sodium chloride and 50.0 mL of 0.200 M sodium sulfate?
-
Corona Company's balance sheet accounts follow: What is Corona Company's days' sales in inventory ratio for 2014, assuming net sales and gross profit for the period were $1,706,786, $1,122,812...
-
A monopolist produces sets/boxes of golf balls. Assume that the demand for a set of golf balls is P=100-Q and its MC=20. Suppose the monopolist sets a two-part tariff (a per unit fee and a lump sum...
-
To demonstrate competency in this unit, a person must: Call an Auction Instructions in second document titled Auction Script Guide Execute the contract for the successful bidder This can be a...
-
3. Customers arrive at a two-server service station according to a Poisson process with rate A. Whenever a new customer arrives, any customer in the system immediately departs. A new arrival enters...
-
Question 8 A national survey of 600 Formula One fans was conducted to learn if they can afford the Austin Cota F1 race tickets. Use the data from the excel file to solve the following. What's the...
-
Could you please check and send me the last results, because the system announced the wrong answer. Thanks Question 1 George was offered two options for a car he was purchasing: Lease option: Pay...
-
Here's a strategy an algebra student discovered for finding the monthly payment you would need to make to pay off a loan. First, specify the loan amount, the annual interest rate, and the term...
-
Would you use the adjacency matrix structure or the adjacency list structure in each of the following cases? Justify your choice. a. The graph has 10,000 vertices and 20,000 edges, and it is...
-
Soft Glow Candle Co. projected sales of 78,000 candles for 2010. The estimated January 1, 2010, inventory is 3,600 units, and the desired December 31, 2010, inventory is 4,500 units. What is the...
-
Day Timer Publishers Inc. projected sales of 205,000 schedule planners for 2010. The estimated January 1, 2010, inventory is 18,500 units, and the desired December 31, 2010, inventory is 15,000...
-
Soft Glow Candle Co, budgeted production of 78,900 candles in 2010. Wax is required to produce a candle. Assume 8 ounces (one half of a pound) of wax is required for each candle. The estimated...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


