Balance each of the following chemical equations by inspection. (a) Sn(s) + P(s) Sn3P(s) (b) Fe(CO3)3(s)
Question:
Balance each of the following chemical equations by inspection.
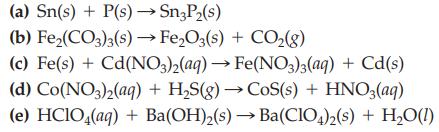
Transcribed Image Text:
(a) Sn(s) + P(s) →→→Sn3P₂(s) (b) Fe₂(CO3)3(s) → Fe₂O3(s) + CO₂(g) (c) Fe(s) + Cd(NO3)2(aq) → Fe(NO3)3(aq) + Cd(s) (d) Co(NO3)2(aq) + H₂S(g) → CoS(s) + HNO3(aq) (e) HClO4(aq) + Ba(OH)₂(s)→ Ba(ClO4)2(s) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Equation a Sns Ps Sn3P2s There is one tin Sn atom on the left and three on the rightso multiply Sn o...View the full answer

Answered By

Poonam Chaudhary
I have 15 month+ Teaching Experience
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following chemical equations by inspection. (a) PCl 5 (s) + H 2 O(l) H 3 PO 4 (aq) + HCl(aq) (b) TiCl 4 (s) + H 2 O(g) TiO 2 (s) + HCl(g).
-
Balance each of the following chemical equations by inspection. (a) F 2 (g) + NaBr(aq) Br 2 (l) + NaF(aq) (b) Sb 2 S 3 (s) + HCl(aq) SbCl 3 (aq) + H 2 S(g).
-
Balance each of the following chemical equations by inspection. (a) FeO(l) + Al(l) Al 2 O 3 (l) + Fe(l) (b) MnO 2 (l) + Al(l) Al 2 O 3 (l) + Mn(l).
-
Aoslia is a small country that takes the world price of corn as given. Its domestic supply and demand for corn are given by the following: a. Assume initially that Aoslia does not open to trade. What...
-
Mindy Kaliny Company has the following internal control procedures over cash disbursements. Identify the internal control principle that is applicable to each procedure. 1. Company checks are...
-
Explain how measures such as GDP, PPP (Big Mac Index), and HDI help marketers decide whether to enter a global market. What are the weaknesses of these measures?
-
Which of the following describes an IASB requirement that the FASB has adopted as part of the short-term convergence project? LO4 a. Following the IASB format for presentation of a statement of...
-
The Sterling Trust owns a business and generated $100,000 in depreciation deductions for the tax year. Mona is one of the income beneficiaries of the entity. Given the following information, can Mona...
-
IFRS vs. GAAP: Highlight key differences between International Financial Reporting Standards (IFRS) and Generally Accepted Accounting Principles (GAAP), and discuss the implications for multinational...
-
Classify each reaction in Exercise 17 as one of the following: combination, decomposition, single replacement, double replacement, or neutralization. Exercise 17 Balance each of the following...
-
Balance each of the following chemical equations by inspection (a) Pb(s) + O(g) PbO(s) (b) LINO3(s)LiNO(s) + O(g) (c) Mg(s) + HCHO(aq) Mg(CHO)2(aq) + H(g) (d) Hg2(NO3)2(aq) + NaBr(aq) HgBr(s) +...
-
You have been requested to recommend one of the mutually exclusive industrial sanitation control systems that are given below. If MARR is 15% per year, which system would you select? Use the B-C...
-
10.) Steam enters a well-insulated turbine at 6 MPa, 400C and expands to 200 kPa, saturated vapor at a rate of 10 kg/s. (a) Draw a schematic of the process (5 pts). (b) Determine the exergy...
-
4. [8 marks] The tides in the Bay of Fundy are some of the largest in the world. The height, h(t), of the tide in meters after t hourse can be modeled by 39 h(t) = 25 con (77) + 30 4 COS 6 (a) What...
-
Wolfe, Inc. had credit sales for the period of $144,000. The balance in Allowance for Doubtful Accounts is a debit of $653. If Wolfe estimates that 2% of credit sales will be uncollectible, what is...
-
Water at 20C is to be pumped from a reservoir (ZA = 5 m) to another reservoir at a higher elevation (ZB = 13 m) through two 36-m- long pipes connected in parallel as shown. The pipes are made of...
-
Delph Company uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 53,000 machine-hours would...
-
For an electron in a certain rectangular well with a depth of 20.0 eV, the lowest energy level lies 3.00 eV above the bottom of the well. Find the width of this well.
-
Modify the counter from Exercise 5.44 such that the counter will either increment by 4 or load a new 32-bit value, D, on each clock edge, depending on a control signal Load. When Load = 1, the...
-
Depletion ComputationsTimber Jonas Lumber Company owns a 7,000-acre tract of timber purchased in 2003 at a cost of $1,300 per acre. At the time of purchase the land was estimated to have a value of...
-
Depletion ComputationsMining Henrik Mining Company purchased land on February 1, 2010, at a cost of $1,250,000. It estimated that a total of 60,000 tons of mineral was available for mining. After it...
-
Depletion ComputationsMinerals At the beginning of 2010, Callaway Company acquired a mine for $850,000. Of this amount, $100,000 was ascribed to the land value and the remaining portion to the...
-
Problem Set Time Value of Money 1. In 10 years, what is the value of $100 invested today at an interest rate of 8% per year, compounded annually? 2. In 10 years, what is the value of $100 invested...
-
The Blending Department of Luongo Company has the following cost and production data for the month of April. Costs: Work in process, April 1 Direct materials: 100% complete $120,000 Conversion costs:...
-
Q3 plz answer correctly and check work Builtrite's upper management has been comparing their books to industry standards and came up with the following question: Why is our operating profit margin...

Study smarter with the SolutionInn App


