Balance each of the following chemical equations by inspection. (a) Co(s) + O(g) CoO3(s) (b) LiClO3(s)
Question:
Balance each of the following chemical equations by inspection.
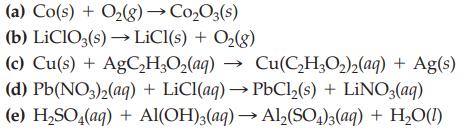
Transcribed Image Text:
(a) Co(s) + O₂(g) → Co₂O3(s) (b) LiClO3(s) → LiCl(s) + O₂(g) (c) Cu(s) + AgC₂H₂O₂(aq) → Cu(C₂H₂O₂)2(aq) + Ag(s) (d) Pb(NO3)2(aq) + LiCl(aq) →PbCl₂(s) + LiNO3(aq) (e) H₂SO4(aq) + Al(OH)3(aq) → Al₂(SO4)3(aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Cos Og CoOs This equation is already balanced There are 1 cobalt atom on each side 2 oxygen atoms ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following chemical equations by inspection. (a) PCl 5 (s) + H 2 O(l) H 3 PO 4 (aq) + HCl(aq) (b) TiCl 4 (s) + H 2 O(g) TiO 2 (s) + HCl(g).
-
Balance each of the following chemical equations by inspection. (a) F 2 (g) + NaBr(aq) Br 2 (l) + NaF(aq) (b) Sb 2 S 3 (s) + HCl(aq) SbCl 3 (aq) + H 2 S(g).
-
Balance each of the following chemical equations by inspection. (a) FeO(l) + Al(l) Al 2 O 3 (l) + Fe(l) (b) MnO 2 (l) + Al(l) Al 2 O 3 (l) + Mn(l).
-
You bought a share of 3.4 percent preferred stock for $96.82 last year. The market price for your stock is now $98.34. What is your total return for last year?
-
The cash register tape for Kelly Kapoor Industries reported sales of $6,891.50. Record the journal entry that would be necessary for each of the following situations. (a) Cash to be accounted for...
-
In 2008, Ellen purchased a house for $150,000 to use as her personal residence. She paid $30,000 and borrowed $120,000 from the local savings and loan company. In 2010 she paid $20,000 to add a room...
-
IAS 1, Presentation of Financial Statements, does not provide guidance with respect to which of the following? LO4 a. The statements that must be included in a complete set of financial statements....
-
Using the information provided here for the Airport Enterprise Fund of the City of Demere, prepare a statement of revenues, expenses, and changes in fund net position for 20X3. Charges for services ....
-
Suppose that a 1 -year zero-coupon bond with face value $100 currently sells at $94.34, while a 2-year zero sells at $84.99. You are considering the purchase of a 2-year-maturity bond making annual...
-
Balance each of the following chemical equations by inspection (a) Pb(s) + O(g) PbO(s) (b) LINO3(s)LiNO(s) + O(g) (c) Mg(s) + HCHO(aq) Mg(CHO)2(aq) + H(g) (d) Hg2(NO3)2(aq) + NaBr(aq) HgBr(s) +...
-
Write a balanced chemical equation for each of the following neutralization reactions: (a) Chloric acid neutralizes a strontium hydroxide solution. (b) Phosphoric acid neutralizes a sodium hydroxide...
-
On January 1, 2013, Down Under, Inc. decided to change from the LIFO method of inventory valuation to the FIFO method. The net income (using LIFO) for the four years Down Under has been in business...
-
Consider a system consisting of a colloidal particle of radius and charge Q-+20e (e is the charge of an electron) stationary in the center of a spherical cavity of radius R=5. Its counterions have...
-
Use the Empirical Rule to determine the percentage of candies with weights between 0.7 and 0.98 gram. Hint: x=0.84.
-
A sample of 16 items provides a sample standard deviation of 9.5. Test the following hypotheses using a = .05. Ho: 0250 2 Ha > 50 a. Calculate the value of the test statistic (to 2 decimals). 27.08...
-
During May, Darling Company incurred factory overhead costs as follows: indirect materials, $1,170; indirect labor, $2,000; utilities cost, $1,270; and factory depreciation, $5,850. Journalize the...
-
Practice 1 Let f(0) = cos(0). For each interval in the table below, determine the characteristics of f(e) Positive or negative Increasing or decreasing Concave up or concave down Let g(0) = 00
-
Sketch ( for the next-lowest bound level in Fig. 2.5 II 3D0 3D1
-
Catalytic hydrogenation of naphthalene over PdC results in rapid addition of 2 moles of H 2 . Propose a structure for this product.
-
Depreciation for Partial PeriodSL, SYD, and DDB Alladin Company purchased Machine #201 on May 1, 2010. The following information relating to Machine #201 was gathered at the end of May....
-
Depreciation for Partial PeriodsSL, Act, SYD, and DDB the cost of equipment purchased by Charleston, Inc., on June 1, 2010 is $89,000. It is estimated that the machine will have a $5,000 salvage...
-
Depreciation?SYD, Act., SL, and DDB the following data relate to the Plant Assets account of Eshkol, Inc. at December 31, 2010. *In the year an asset is purchased, Eshkol, Inc. does not record any...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


