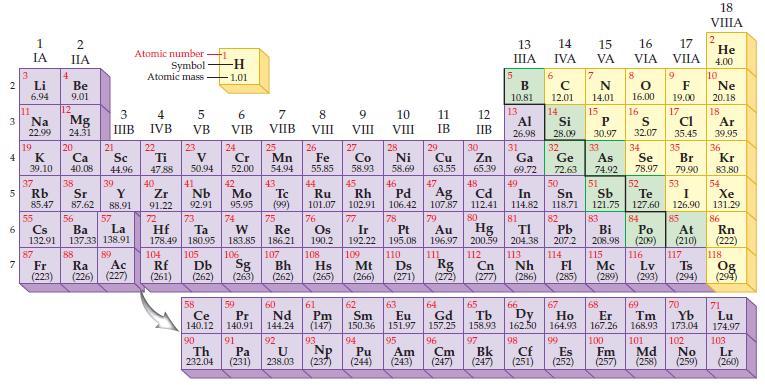
Based on the general trends in the periodic table, predict which element in each of the following
Question:
Based on the general trends in the periodic table, predict which element in each of the following pairs has the most metallic character:
(a) Sn or Pb
(b) Ag or Sr
(c) Al or B
(d) Ga or Ge.
Periodic Table:
Transcribed Image Text:
2 3 4 5 10 6 7 3 11 Li 6.94 1 IA Na 22.99 19 K 39.10 37 87 4 Fr (223) 2 IIA Be 9.01 12 Mg 24.31 20 Ca 40.08 38 21 Rb Sr Y 85.47 87.62 88.91 88 3 IIIB Sc 44.96 39 55 56 La Cs Ba 132.91 137.33 138.91 57 89 Ra Ac (226) (227) Atomic number Symbol Atomic mass 4 IVB 22 Ti 47.88 40 Zr 2 91.22 72 5 VB 104 23 V 50.94 41 73 Hf Ta 178.49 180.95 105 Rf Db (261) (262) -H -1.01 6 VIB Nb Mo 92.91 95.95 90 24 Cr 52.00 42 74 106 Sg (263) 58 Pr Ce 140.12 140.91 91 59 7 VIIB Th Pa 232.04 (231) 25 W Re 183.85 186.21 Mn 54.94 43. Tc (99) 75 107 Bh (262) 60 Nd 144.24 92 U 238.03 8 VIII 26 Fe 55.85 76 Os 190.2 61 Pm (147) 93 9 VIII Np (237) 10 VIII 11 IB 12 IIB 27 28 29 Zn Co Ni Cu 58.93 58.69 63.55 65.39 30 5 44 47 48 49 45 46 Ru Rh Pd Ag Cd In 101.07 102.91 106.42 107.87 112.41 114.82 77 78 79 80 Ir Pt 192.22 195.08 109 110 Hs Mt Ds Rg Cn (265) (266) (271) (272) (277) Au Hg 196.97 200.59 111 112 108 13 ΠΙΑ B 10.81 13 Ga 69.72 6 14 IVA с 12.01 Al Si 26.98 28.09 32 31 Ge 72.63 14 81 82 TI Pb 204.38 207.2 113 114 Nh Fl (286) (285) 15 16 17 VA VIA VILA 66 67 62 63 64 65 Sm Eu Gd Tb Dy Ho 150.36 151.97 157.25 158.93 162.50 164.93 94 95 96 97 Pu Am Cm Bk (244) (243) (247) (247) 7 N 14.01 15 P 30.97 8 68 O 16.00 33 34 50 As Se 74.92 78.97 51 52 Sn Sb Te I 118.71 121.75 127.60 126.90 53 83 84 85 Bi Po At 208.98 (209) (210) 115 116 117 Lv Ts (289) (293) (294) Mc 98 99 167.26 100 Cf Es Fm (251) (252) (257) 16 S 32.07 9 F 19.00 17 Cl 35.45 35 69 70 Er Tm Yb 168.93 173.04 101 102 Md No (258) (259) Br 79.90 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39,95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a P...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the smaller atomic radius: (a) Na or K (b) P or N (c) Ca or Ni (d) Si or S. Periodic Table:...
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy: (a) Li or Na (b) O or F. Periodic Table: 2 3 4 in 6 3 7 Li...
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy: (a) Na or Mg (b) O or S. Periodic Table: 2 3 4 15 6 7 3 11...
-
What is the difference between a Type I error and a Type II error?
-
Performance Measures. Information from the Form 990 for the American Heart Association for the fiscal year ending June 30, 2007, follows. Required a. Compute the following performance measures using...
-
A 13 cm by 13 cm steel angle with the dimensions shown is attached to a wall with a surface temperature of 600 K. The surrounding air is at 300 K, and the convective heat-transfer coefficient between...
-
3 In Figure 86, the dormitory market segment includes students living in college-owned residence halls, sororities, and fraternities. What market needs are common to these students that justify...
-
Journalize the following transactions in the accounts of Pro Medical Co., a medical equipment company that uses the direct write-off method of accounting for uncollectible receivables: Jan. 30. Sold...
-
Contribution Margin Ratio a. Imelda Company budgets sales of $1,800,000, fixed costs of $394,000, and variable costs of $1,116,000. What is the contribution margin ratio for Imelda Company? (Enter...
-
What is the group number for each of the following families of elements? (a) Alkali metals (b) Alkaline earth metals (c) Halogens (d) Noble gases.
-
Why did Mendeleev not include argon in his periodic table of 1871? Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 Cs 132.91 87 4 Fr (223) 2 IIA Be 9.01 12 Mg 24.31...
-
Can you detect any correlation between Taylors approach to motivation and systems of performance-related pay?
-
Show how you would go about balancing the following equations: Cu + HNO3 Cu(NO3)2 + NO + H2O HIO3 + Fel2 + HCI FeCl3 + ICI + H2O 2.Conservation of mass A student places 0.58 g of iron and 1.600 g...
-
Sales MOSS COMPANY Income Statement For Year Ended December 31, 2021 Cost of goods sold Gross profit Operating expenses (excluding depreciation) Depreciation expense Income before taxes Income taxes...
-
Prior to the Covid-19 epidemic, Master's and Ph.D. programs in psychology required applying students to submit their scores on the standardized graduate admission exam (GRE). For the past three...
-
Benicio wants to make sure that the Sales table does not contain any duplicate records, which would make any sales analysis incorrect. Identify and remove duplicate records in the Sales table as...
-
University Car Wash purchased new soap dispensing equipment that cost $261,000 including installation. The company estimates that the equipment will have a residual value of $27,000. University Car...
-
What is the difference between short-run business decisions and long-run strategic plans?
-
(a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing...
-
Where can authoritative iGAAP guidance be found related to cash and receivables?
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to the accounting for cash and receivables.
-
Simonies Company, which uses iGAAP, has a note receivable with a carrying value of $30,000 at December 31, 2010. On January 2, 2011, the borrower declares bankruptcy, and Simonis estimates that only...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


