Based on the general trends in the periodic table, predict which element in each of the following
Question:
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy:
(a) Na or Mg
(b) O or S.
Periodic Table:
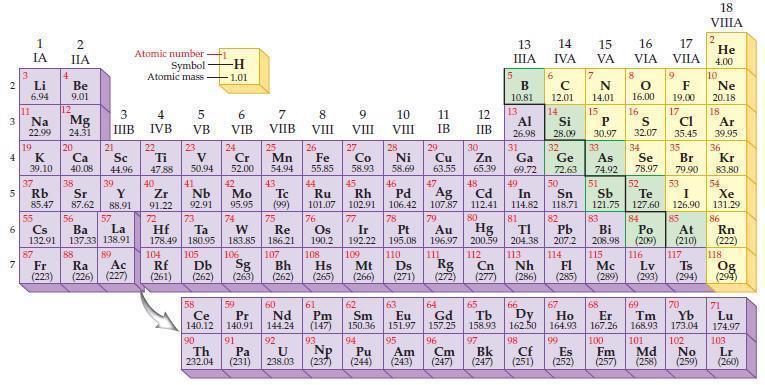
Transcribed Image Text:
2 3 4 15 6 7 3 11 Li 6.94 Na 22.99 19 1 IA 37 R Rb 55 4 87 2 IIA Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 20 38 21 Sr Y 85.47 87.62 88.91 3 IIIB 88 39 57 56 Cs La Ba 132.91 137.33 138.91 89 Fr Ra Ac (223) (226) (227) Atomic number Symbol Atomic mass 4 IVB 22 Ti 47.88 40 Zr 2 91.22 72 5 VB 104 23 V 50.94 41 73 Hf Ta 178.49 180.95 105 Rf Db (261) (262) -H -1.01 6 VIB Nb Mo 92.91 95.95 90 24 Cr 52.00 42 74 106 Sg (263) 58 Pr Ce 140.12 140.91 91 59 7 VIIB Th Pa 232.04 (231) 25 W Re 183.85 186.21 Mn 54.94 43. Tc (99) 75 107 Bh (262) 60 Nd 144.24 92 U 238.03 8 VIII 26 Fe 55.85 76 Os 190.2 61 Pm (147) 93 NP 9 VIII (237) 10 VIII 11 IB 12 IIB 27 28 29 Zn Co Ni Cu 58.93 58.69 63.55 65.39 44 47 48 49 45 46 Ru Rh Pd Ag Cd In 101.07 102.91 106.42 107.87 112.41 114.82 77 78 80 Ir Pt 192.22 195.08 109 110 Hs Mt Ds Rg Cn (265) (266) (271) (272) (277) 79 Au Hg 196.97 200.59 111 112 108 30 5 13 ΠΙΑ B 10.81 13 6 14 15 IVA с 12.01 14 81 82 TI Pb 204.38 207.2 113 114 Nh Fl (286) (285) 17 16 VA VIA VILA 66 67 62 63 64 65 Sm Eu Gd Tb Dy Ho 150.36 151.97 157.25 158.93 162.50 164.93 99 94 95 96 97 Pu Am Cm Bk (244) (243) (247) (247) 7 N 14.01 15 Al Si 31 34 26.98 28.09 32 33 Ga Ge As Se 69.72 72.63 74.92 78.97 50 51 52 Sn Sb Te I 118.71 121.75 127.60 126.90 53 P 30.97 8 O 16.00 68 16 98 167.26 100 Cf Es Fm (251) (252) (257) 9 F 19.00 17 Cl 35.45 S 32.07 35 83 84 85 Bi Po At 208.98 (209) (210) 115 116 117 Mc Lv Ts (289) (293) (294) Br 79.90 69 70 Er Tm Yb 168.93 173.04 101 102 Md No (258) (259) 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39,95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
If $1,000 is put into a savings account at 8% interest compounded continuously, how much will it be worth at the end of 4 years?
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy: (a) Li or Na (b) O or F. Periodic Table: 2 3 4 in 6 3 7 Li...
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the most metallic character: (a) Sn or Pb (b) Ag or Sr (c) Al or B (d) Ga or Ge. Periodic...
-
If possible, completely factor the expressions in Problems 336. 9-x-2x
-
Revenue and Related Transactions. During its current fiscal year, Dearborn General Hospital, a not-for-profit health care organization, had the following revenue-related transactions (amounts...
-
Determine the diameter of the glass tube necessary to keep the capillary-height change of water at 30C less than 1 mm.
-
If you were a foreign firm, would you consider doing business in Indonesia? L01
-
1. What are the advantages and disadvantages of Rovios current business model? 2. Do you agree with the company chairman Kaj Hed when he says he is satisfied with Rovios current situation? Rovio...
-
Consider the following abbreviated financial statements for Parrothead Enterprises: a . What is owners' equity for 2 0 2 0 and 2 0 2 1 ? ( Do not round intermediate calculations and round your answer...
-
Which group in the periodic table has the highest ionization energy? Which group has the lowest ionization energy? Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 Cs...
-
Propose two ways of drawing the electron dot formula for Mg.
-
In 2014, Time Warner, Inc. spun off its wholly owned unit, Time Inc., as an independent, publicly traded company. The spin-off was achieved by distributing new Time Inc. shares to the holders of Time...
-
1. A concise introduction of the brand, including but not limited to a brief history, location information, size of the business, product/service offering, and so on.Give brief explanation. 2. Which...
-
A wood frame structure as shown to the right. The framingconsists of 2x6 studs, a single 2x6 bottom plate, two 2x6 topplates and a 2x10 joist. The studs are spaced at 16 in. on centerand sheathed...
-
In your reflection journal please list your order - 'most efficient' mediums at the top, 'least efficient' at the bottom. (for eg. social media, display ads, etc)Then, in five hundred words or more,...
-
Energy prices and global warming are discussed daily in the news as environmental impact of e-waste is just beginning to be recognized. Sustainability and corporate social responsibility need to be...
-
3. A Channel section is connected to a 10mm gusset plate with 20mm- diameter bolts as shown in the figure. The connecting member is subjected to dead load and live load only. The pitch distance,...
-
Each year, a large clothing store in New York City sends its top five salespersons on a five-day retreat in Orlando, Florida. The retreat begins on the first Monday in February and ends the following...
-
Let (X. A. p) be a measure space. Show that for any A,B A, we have the equality: (AUB)+(An B) = (A) + (B).
-
A lease agreement between Lennox Leasing Company and Gill Company is described in E21-8. (Round all numbers to the nearest cent.) Refer to the data in E21-8 and do the following for the lessor. (a)...
-
Fieval Leasing Company signs an agreement on January 1, 2010, to lease equipment to Reid Company. The following information relates to this agreement. 1. The term of the non-cancelable lease is 6...
-
Grady Leasing Company signs an agreement on January 1, 2010, to lease equipment to Azure Company. The following information relates to this agreement. 1. The term of the non-cancelable lease is 5...
-
help me A 35% discount on 3 smart phone amounts to $385. What is the phone's list price? Answer =$ (rounded to the nearest cent)
-
What effect is there on the income statement and balance sheet when an expense is left too long as a liability
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid

Study smarter with the SolutionInn App


