Calculate the atomic mass for zinc given the following data for its natural isotopes. 64Zn 66Zn 67Zn
Question:
Calculate the atomic mass for zinc given the following data for its natural isotopes.
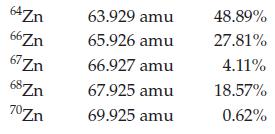
Transcribed Image Text:
64Zn 66Zn 67Zn 68 Zn 70Zn 63.929 amu 65.926 amu 66.927 amu 67.925 amu 69.925 amu 48.89% 27.81% 4.11% 18.57% 0.62%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the atomic mass for zinc we need to multiply the mass of each isotope ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Modify the code to add another attribute and a method that fits (is consistent with) the object the class is modeling, or suggest a different version of the code. Write the main method to demonstrate...
-
Calculate the atomic mass for iron given the following data for its natural isotopes. 54Fe: 56Fe: 57 Fe: 58 Fe: 53.940 amu 55.935 amu 56.935 amu 57.933 amu 5.82% 91.66% 2.19% 0.33%
-
Calculate the atomic mass for magnesium given the following data for its natural isotopes. 24 Mg: 25Mg: 26Mg: 23.985 amu 24.986 amu 25.983 amu 78.70% 10.13% 11.17%
-
In Exercises 1126, determine whether each equation defines y as a function of x. x + y = 16
-
If volume is increasing, would a company benefit more from a pure variable or a pure fixed cost structure? Which cost structure would be advantageous if volume is decreasing?
-
You have recently been hired by Keafer Manufacturing to work in its newly established treasury department. Keafer Manufacturing is a small company that produces highly customized cardboard boxes in a...
-
Suppose you have developed a regression model to explain the relationship between y and x1, x2, and x3. The ranges of the variables you observed were as follows: 50 y 100, 10 x1 75, .25 x2 .75,...
-
Differing Frames of Reference: E-Mail Cross-Cultural Misunderstanding A cultural misunderstanding nearly derailed an Indo-Japanese bridge-building project. An Indian firm sent a detailed list of...
-
Private insurance companies may not successfully prevent panic of bank runs because _____ The size of the funds is more than enough to pay all depositors They overcharge the institutions on their...
-
Which has the longer wavelength: red light or yellow light?
-
Calculate the atomic mass for lithium given the following data for its natural isotopes. 'Li: 7Li: 6.015 amu 7.016 amu 7.42% 92.58%
-
Solve the equations in Problems 2748. x - 6x +7=0
-
Gordon Rivers, the city manager of Saratoga, Florida, pitched the proposed design schedule back at Jay Andrews. Jay Andrews is the project manager for Major Design Corporation (MDC). The city of...
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts straight to retained earnings. The balance of \(\$ 8,500\) in the retained earnings account...
-
Draw a Keynesian cross diagram to show the effects of a rise in autonomous expenditure on an economy operating below full employment output.
-
Governments in many countries are acutely aware of the environmental problems that vehicle emissions can have. Many car manufacturers are exploring the production of electric vehicles, but production...
-
Draw a simple diagram of John Woodens pyramid of success. You can find it at the official Wooden website www.coachwooden.com/index2.html.
-
a. Draw all the isomers with molecular formula that contain a cyclobutane ring. (hint: there are seven.) b. Name the compounds without specifying the configuration of any asymmetric carbons. C. c....
-
A superior criticized a sales manager for selling high-revenue, low-profit items instead of lower-revenue but higher-profit items. The sales manager responded, My income is based on commissions that...
-
Without actually doing any calculations, describe how the preliminary offering range for the price of an IPO would be determined? MINI CASE Randys, a family-owned restaurant chain operating in...
-
What is a road show? What is book building? MINI CASE Randys, a family-owned restaurant chain operating in Alabama, has grown to the point where expansion throughout the entire southeast is feasible....
-
Describe the typical first-day returns of an IPO and the long-term returns to IPO investors. MINI CASE Randys, a family-owned restaurant chain operating in Alabama, has grown to the point where...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


